KEY POINTS
1. Prior to cannulation for cardiopulmonary bypass (CPB), the anesthesiologist must assure that adequate heparin-induced anticoagulation has been achieved, which is typically diagnosed as an activated clotting time (ACT) >400 s.
2. At commencement of CPB, the anesthesiologist should confirm full bypass (typically by loss of pulsatile arterial waveform), discontinue mechanical ventilation, and assess adequacy of perfusion pressure and flow. Other tasks include assessment of adequacy of anesthesia and neuromuscular blockade, emptying of urine, withdrawing the pulmonary artery (PA) catheter to the proximal PA, and assessment of adequacy of other monitors such as central venous pressure (CVP), electrocardiogram (ECG), and temperature measurement devices.
3. During cardioplegic arrest, the anesthesiologist monitors the adequacy of left ventricular emptying via direct observation of the heart, by ensuring of low cardiac filling pressures, and by the presence of electrical silence on the ECG.
4. Anesthesia during CPB can be maintained with various combinations of volatile agents, opioids, and hypnotic agents (e.g., propofol, midazolam). Especially during hypothermia, maintaining neuromuscular blockade is important in order to avoid spontaneous breathing and visible or subclinical shivering. Anesthetic requirements are reduced during hypothermia.
5. Appropriate perfusion flows and pressures during CPB are controversial, but for most patients a normothermic perfusion index of 2.4 L/min/M2 and mean arterial pressures (MAPs) of 50 to 70 mm Hg suffice. Continuous monitoring of mixed venous oxygen saturation is useful to assess global perfusion adequacy, as are intermittent arterial blood gas measurements.
6. Moderate hemodilution is useful during CPB, as aided by clear CPB circuit priming solutions. Minimum safe hemoglobin (Hb) concentrations during CPB are controversial, but for most patients Hb ≥ 6.5 g/dL (hematocrit [Hct] ≥ 20%) is safe in the absence of evidence for inadequate oxygen delivery (e.g., metabolic acidosis, low SVO2).
7. Hypothermia is commonly used during CPB to reduce oxygen consumption and metabolism and to confer organ protection. Often temperatures of 32 to 34°C are used in combination with alpha-stat arterial blood gas management. Rewarming should be accomplished slowly and should not proceed beyond a core temperature of 37°C.
8. Cardioplegic solutions have a variety of “recipes,” but most contain a hyperkalemic solution at a low temperature as well as a combination of crystalloid and blood. Cardioplegia can be administered in an antegrade (via coronary arteries) or retrograde (via coronary sinus) direction.
9. CPB can produce catastrophic events such as aortic dissection, cerebral ischemia from aortic cannula malposition, regional venous congestion from venous cannula malposition, venous obstruction from air lock, massive air embolism, pump or oxygenator failure, and blood clots in the extracorporeal circuit.
10. A variety of unusual conditions such as sickle cell disease, cold agglutinin disease, malignant hyperthermia (MH), and angioedema may present during or influence the management of CPB.

I. Preparations for CPB
This requires close communication and coordination between surgeon, perfusionist, and anesthesiologist.
A. Assembling and checking the CPB circuit
The perfusionist assembles the CPB circuit (Fig. 8.1) before commencement of surgery, so that CPB can be instituted rapidly if necessary. The circuit components [e.g., pump (roller or centrifugal), tubing (e.g., standard or heparin-bonded), reservoir (venous and possibly arterial), oxygenator, filters, and safety monitors] are usually decided by institutional preference, but should comply with guidelines of professional organizations. Similarly, the type and volume of CPB prime are decided by the perfusionist in consultation with surgeon and anesthesiologist. The perfusionist checks all components using an approved checklist. See Chapter 21 for details on CPB circuit design and use.
B. Anesthesiologist pre-CPB checklist (Table 8.1)
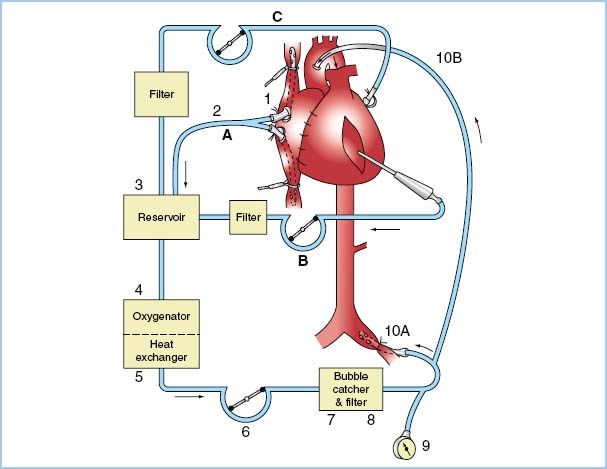
Figure 8.1 CPB circuit (example). Blood drains by gravity (or with vacuum assistance) (A) from venae cavae (1) through venous cannula (2) into venous reservoir (3). Blood from surgical field suction and from vent is pumped (B, C) into cardiotomy reservoir (not shown) and then drains into venous reservoir (3). Venous blood is oxygenated (4), temperature adjusted (5), raised to arterial pressure (6), filtered (7, 8), and injected into either aorta (10B) or femoral artery (10A). Arterial line pressure is monitored (9). Note that items 3, 4, and 5 often are single integral units. (Modified from Nose Y. The Oxygenator. St. Louis, MO: Mosby; 1973: 53.)
1
A separate pre-CPB checklist is undertaken by the anesthesiologist (Table 8.1). This includes ensuring that anticoagulation is sufficient for cannulation and CPB (e.g., ACT > 400 s), adequate anesthesia will be provided during CPB, fluid infusions are ceased, and monitors are withdrawn to safe positions for CPB (e.g., Swan Ganz catheter, if present, is withdrawn into the proximal PA and a transesophageal echocardiography [TEE] probe, if present, is returned to a neutral position within the esophagus). This is also an appropriate time to empty the urinary catheter drainage bag, and to check the patient’s face and pupils so that any changes occurring as a result of CPB can be recognized.
Table 8.1 Prebypass checklist

C. Management of arterial and venous cannulation
In most cases, the arterial cannulation site will be the distal ascending aorta. Prior to cannulation, adequate anticoagulation must be confirmed, and nitrous oxide, if used, must be ceased (to avoid the expansion of any air bubbles inadvertently introduced during cannulation). The anesthesiologist typically reduces the systemic systolic blood pressure to about 80 to 100 mm Hg to reduce the risk of arterial dissection while the cannula is placed. The perfusionist then can check that the pressure trace through the arterial cannula matches the systemic blood pressure trace, which ensures that the arterial cannula has been placed within the aortic lumen. If a two-stage venous cannula is selected, the surgeon then inserts the venous cannula into the right atrium and guides the distal stage of the cannula into the inferior vena cava. A separate smaller cannula is guided via the right atrium into the coronary sinus if retrograde cardioplegia is planned. Femoral arterial cannulation may be used if access to the distal ascending aorta is limited, but axillary artery cannulation has become popular in this situation, because this avoids retrograde flow in the often-atherosclerotic thoracic and abdominal aorta. The venous cannula can also be inserted through a femoral vein if necessary, but must be advanced to the right atrium for adequate drainage. In these cases, TEE is required to confirm satisfactory venous cannula position.
II. Commencement of CPB
A. Establishing “full” flow
Once the cannulas are in place and all other checks and observations are satisfactory, the surgeon indicates that CPB should commence. The perfusionist gradually increases the flow of oxygenated blood through the arterial cannula into the systemic circulation. If an arterial cannula clamp is present, this must first be released. At the same time, the venous clamp is gradually released, allowing an increasing proportion of systemic venous blood to drain into the CPB reservoir. Care is taken to match the arterial flow to the venous drainage. Typically, the arterial inflow is increased to equate to a normal cardiac output (CO) for the patient over about 30 to 60 s. The “normal” CO is usually based on a cardiac index of about 2.4 L/min/M2. This is known as “full flow.” At this stage the left ventricle (LV) will have ceased to eject, and the CVP will be close to zero.
2
B. Initial bypass checklist (Table 8.2)
Table 8.2 Initial Cardiopulmonary bypass checklist
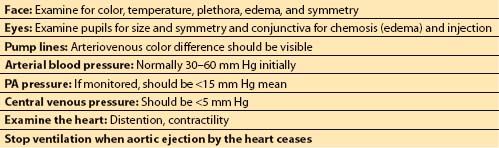
As CPB commences, the anesthesiologist should check the patient’s face for asymmetry of color and the patient’s pupils for asymmetry of size. Satisfactory oxygenator function should be confirmed by checking the color of the arterial blood and, if available, the in-line PaO2 or oxygen saturation monitor. Adequate venous drainage is confirmed by the absence of pulsatility in the arterial waveform and low CVP (typically <5 mm Hg).
C. Cessation of ventilation
If the observations of the initial CPB checklist are satisfactory and full flow is established, ventilation of the lungs is ceased, and the airway pressure release valve is opened fully to avoid inflation of the lungs. It is not necessary to disconnect the anesthesia circuit from the anesthesia machine.
3
D. Monitoring. Patient monitoring during CPB includes continuous ECG, MAP, CVP, core temperature (e.g., nasopharyngeal, tympanic membrane, bladder), blood temperature, and urine output. Continuous monitoring of arterial and venous oxygen saturations and in-line monitoring of arterial blood gases, pH, electrolytes, and Hct are also recommended. Measurement errors may lead to inappropriate management with potentially disastrous consequences, so frequent checks confirming accuracy are advised. Intermittent monitoring of coagulation (e.g., ACT), laboratory arterial blood gases, electrolytes (including calcium, potassium, blood glucose, and possibly lactate), and Hb are also appropriate. Estimates of Hb and blood glucose can be obtained rapidly using point-of-care devices.
III. Typical CPB sequence
A. Typical coronary artery bypass graft (CABG) operation. A typical CABG operation proceeds as follows. Total CPB is initiated and mild-to-moderate hypothermia is either actively induced (30 to 34°C) or permitted to occur passively (sometimes called “drifting”). The aorta is cross-clamped and cardioplegic solution is infused antegrade through the aortic root and/or retrograde via the coronary sinus to arrest the heart. The distal saphenous vein grafts are placed on the most severely diseased coronary arteries first, to facilitate administration of additional cardioplegic solution (via the vein graft) distal to the stenoses. The internal mammary artery anastomosis (if used) is often constructed last because of its fragility and shorter length. Rewarming typically begins when the final distal anastomosis is started. The aorta is unclamped and either an aortic side clamp is applied or an internal occlusive device is used to permit construction of proximal vein graft anastomoses while cardioplegic solution is being washed out of the heart. When it is sufficiently warm, the heart is defibrillated if necessary. Alternatively, the proximal anastomoses are completed with the aortic clamp in place, in order to reduce instrumentation of the aorta (with the risk of dislodging of atheroma). Total CPB continues until the heart is reperfused from its new blood supply. Finally, when the patient is adequately rewarmed and the coronary artery grafts are completed, epicardial pacing wires are placed, and CPB is then terminated.
B. Typical aortic valve replacement or repair operation. After initiation of CPB and application of the aortic cross-clamp, the aortic root is opened, and cardioplegic solution is infused into each coronary ostium under direct vision (to prevent retrograde filling of the LV with cardioplegic solution through an incompetent aortic valve). Commonly, cardioplegia is administered retrograde via the coronary sinus either instead of or in addition to antegrade cardioplegia. The valve is repaired or replaced. Rewarming commences toward the end of valve replacement. The heart is irrigated to remove air or tissue debris, and the aortotomy is closed except for a vent. The aortic cross-clamp is removed (often with the patient in a head-down position) and the heart is defibrillated if necessary. Final de-airing occurs as venous drainage to the pump is retarded, the heart fills and begins to eject (partial CPB), and air is aspirated through the aortic vent, an LV vent, or a needle placed in the apex of the heart. During de-airing, the lungs are inflated to help flush bubbles out of pulmonary veins and the heart chambers, and TEE is viewed to monitor air evacuation.
C. Typical mitral valve replacement or repair operation. This operation is similar to aortic valve surgery (see Section 2 above), except that the left atrium (or right atrium for a trans-atrial septal approach) is opened instead of the aorta and the cardioplegia infusion can take place through the aortic root and the coronary sinus. The valve is replaced or repaired, and a large vent tube is passed through the mitral valve into the LV to prevent ejection of blood into the aorta until de-airing is completed. After thorough irrigation of the field and closure of the atriotomy except for the LV vent, the aortic cross-clamp is removed, often with the patient in a head-down position. The heart is defibrillated if necessary, and de-airing occurs as described above. Finally, the LV vent is removed, and de-airing is completed.
D. Typical combined valve–CABG operation. Usually the distal vein–graft anastomoses are created first, to permit cardioplegia of the myocardium distal to severe coronary stenoses. Also, lifting the heart to access the posterior wall vessels can disrupt myocardium if an artificial valve has been inserted, especially in the case of mitral valve replacement. Next, the valve is operated on, and the operation proceeds as described above.
IV. Maintenance of CPB
A. Anesthesia
1. Choice of agent and technique. Just as in the pre-CPB period, anesthesia is typically provided by a potent volatile agent or an infusion of intravenous anesthetic (e.g., propofol) on a background of opiates (e.g., fentanyl, sufentanil) and other sedative drugs (e.g., benzodiazepines). Volatile agents have a more defined role in myocardial protection than other anesthetics through ischemic preconditioning and reduction of reperfusion injury [1].
2. Potent volatile agent via pump oxygenator. This requires a vaporizer mount in the gas inlet line to the oxygenator. A flow- and temperature-compensated vaporizer, often containing isoflurane, is then attached to the mount. The concentration of agent is typically 0.5 to 1.0 MAC at normothermia, depending on the amount of supplementary opiates and sedatives, and is reduced with hypothermia. With most oxygenators, uptake and elimination of the volatile agent are more rapid than that observed via an anesthesia machine, breathing circuit, and normal lungs and heart. Volatile agent administration can be confirmed by disconnecting the gas analysis line from the airway circuit and reconnecting it to the oxygenator outlet [2]. If volatile agents are used, appropriate scavenging of the oxygenator outlet should be ensured. Nitrous oxide is never used, because of its propensity to enlarge gas-filled spaces, including micro- and macro-gas emboli.
4
3. Total intravenous anesthesia. Total intravenous anesthesia (TIVA) can be provided during CPB using a combination of opiates and sedatives, either by intermittent bolus or by infusion. For propofol, the typical infusion rates are 3 to 6 mg/kg/h or a target plasma concentration of 2 to 4 μg/mL, depending on the use of other IV agents and the patient’s temperature. The advantages of TIVA are simplicity, less myocardial depression, and the absence of a need for oxygenator scavenging. However, as with all forms of TIVA, ensuring adequate depth is more difficult, providing greater justification for anesthesia depth monitoring (e.g., bispectral index, entropy) [3,4].
4. Muscle relaxation. Movement of the patient during CPB risks cannula dislodgement and should be avoided. If additional muscle relaxants are not used, adequate anesthesia to prevent movement must be ensured. As in the pre-CPB period, train-of-four monitoring can be titrated to a level of approximately one twitch. Similarly, spontaneous breathing must be avoided, as this risks the development of negative vascular pressures and potential air entrainment.
5. Effect of temperature. Anesthetic requirements fall as temperature drops. However, due to its relatively high blood supply, brain temperature changes faster than core temperature. For this reason, particular care should be taken to ensure adequate anesthesia as soon as rewarming commences, and additional opiates or sedatives may be required. When the patient is normothermic, anesthetic requirements are the same as the pre-CPB phase, although the context-sensitive half-time for most anesthetic drugs increases substantially during and after CPB.
6. Monitoring anesthetic depth. Awareness may be difficult to exclude clinically due to the use of high-dose opiates, cardiovascular drugs (e.g., β-adrenergic blockers), and muscle relaxants. Moreover, hemodynamic cues cannot be used during CPB. The patient should be checked for pupillary dilation and sweating, but these signs may be affected by opiate medication and rewarming. Therefore, emphasis should be placed on ensuring delivery of adequate anesthesia, or the use of depth-of-anesthesia monitors [3,4].
7. Altered pharmacokinetics and pharmacodynamics. The onset of CPB increases the circulating blood volume by the amount of the priming solution for the extracorporeal circuit, but the percentage change in the total volume of distribution of most anesthetic agents is minimal. Neuromuscular blockers constitute an exception to this; hence, they may require supplementation at the onset of CPB. Hemodilution reduces the concentration of plasma proteins, increasing the unbound active proportion of many drugs (e.g., propofol) to offset the reduced total plasma concentration induced by the increased circulating blood volume [5]. A small proportion of some agents (e.g., fentanyl, nitroglycerin) may be absorbed onto the foreign surfaces of the CPB circuit. Hypothermia reduces the rate of drug metabolism and elimination, as does reduction in blood flow to the liver and kidneys. Bypassing the lungs reduces pulmonary metabolism and sequestration of certain drugs and hormones. Reduced blood supply to vessel-poor tissues such as muscle and fat may result in sequestration of drugs given pre-CPB. The response to drugs may also be altered by hypothermia and hemodynamic alterations associated with CPB. The combined effect of these pharmacological changes may be difficult to predict, so the principle of titrating drugs to achieve a certain endpoint is particularly important during CPB.
B. Hemodynamic management. See also Chapter 19.
1. Systemic perfusion flow rate. The most fundamental hemodynamic change during CPB is the generation of the CO by the CPB pump rather than the patient’s heart. The perfusionist regulates the CPB pump to deliver the desired perfusion flow rate for the patient. This is usually based on a nomogram taking into consideration the patient’s height and weight and the core temperature. Typically the perfusion flow rate is set to deliver an effective perfusion flow rate of 2.4 L/min/M2 at 37∞C and about 1.5 L/min/M2 at 28∞C. The amount delivered by the CPB pump is usually set slightly higher than the target flow rate to account for any recirculation within the CPB circuit. For example, a continuous flow of about 200 mL/min from the arterial line filter may be returned to the reservoir through a purge line to provide a mechanism for purging trapped microbubbles. An inadequate perfusion flow rate will result in a low venous Hb oxygen saturation (continuously monitored in the CPB venous return), and the development of a metabolic acidosis due to anaerobic metabolism and the accumulation of lactic acid. If other causes for a low venous Hb oxygen saturation can be excluded (e.g., excessive hemodilution, inadequate anesthesia, over-rewarming to increase metabolism), the perfusion flow rate should be increased accordingly. Unfortunately, a normal venous oxygen saturation does not confirm adequate perfusion of all tissues. Shunting may occur leaving some tissue beds underperfused. An increased metabolic rate due to shivering, which may be subclinical during hypothermia, or to much more unlikely causes such as thyrotoxicosis or MH, may also reduce venous HbO2 saturations despite normal flow rates.
5
2. MAP. The optimum MAP during CPB is not known. Systolic and diastolic pressures are generally of no concern, because the vast majority of CPB is conducted using nonpulsatile flow. If an adequate perfusion flow rate is delivered, the MAP may be irrelevant, so long as the limits of autoregulation have not been exceeded, and also there is no critical stenosis in the arterial supply to individual organs. A higher MAP than necessary should be avoided to reduce noncoronary collateral blood flow (which may wash out cardioplegia). In adults, a conservative approach is usually taken, maintaining the MAP between 50 and 70 mm Hg. Higher levels may be required in patients with pre-existing hypertension or known cerebrovascular disease. Lower levels may be tolerated in children. This range of MAP assumes a CVP < 5 mm Hg. The possibility of measurement error due to inappropriate position of the pressure transducers or zero drift should be checked frequently.
3. Hypotension. The most important consideration in the management of hypotension is to ensure that an adequate perfusion flow rate is being delivered. While a transient reduction of perfusion flow rate (such as may be requested by the surgeon at particular stages of the procedure) is of little consequence, sustained reductions must be avoided. Once adequate perfusion flow rate is confirmed, the MAP may be corrected by increasing the systemic vascular resistance (SVR) with the use of vasoconstrictors such as phenylephrine (0.5 to 10 μg/kg/min, or noradrenaline 0.03 to 0.3 μg/kg/min), on the basis of the following relationship:
SVR = (MAP – CVP)/effective perfusion flow rate (L/min)
where MAP is expressed in mm Hg, CVP in mm Hg, and SVR in mm Hg/L/min (to convert to dyne.s.cm-5, multiply by 80).
As there is substantial individual variability in response to vasoconstrictors, especially during CPB, the dose should be titrated, commencing with less potent agents (e.g., phenylephrine) or smaller doses, and progressing to higher doses of more potent agents (e.g., noradrenaline) if required. Occasionally vasopressin (e.g., 0.01 to 0.05 units/min) is required. The perfusion flow rate can be increased above normal to correct hypotension temporarily (e.g., while vasoconstrictors take effect), but this is not an appropriate strategy to correct persistent hypotension. The onset of CPB is typically associated with sudden hemodilution, which decreases SVR. Cardioplegia solution entering the circulation also reduces SVR and is a common cause of hypotension. Reperfusion of the myocardium after release of the aortic cross-clamp is another common cause of transient hypotension. For these reasons, the use of vasoconstrictors during CPB is common. (Reduced SVR constitutes an oft-underutilized opportunity for communication between the perfusionist and the anesthesia team. Perfusionists can at times create a “roller coaster” with frequent intermittent boluses of phenylephrine at times when MAP management would be smoother if the anesthesiologist would initiate a continuous phenylephrine infusion.)
4. Hypertension. Hypertension is usually the result of an increase in SVR, which may be due to endogenous sympathetic stimulation or hypothermia. Before treating hypertension with direct vasodilators (e.g., nitroglycerin 0.1 to 10 μg/kg/min, sodium nitroprusside 0.1 to 2 μg/kg/min, nicardipine 2 to 5 mg/h), adequate anesthesia should be ensured. Artifactual hypertension due to aortic cannula malposition should also be excluded (see I.C and VII.A). The perfusion flow rate can be decreased below normal to correct hypertension temporarily (e.g., while vasodilators take effect), but not to correct persistent hypertension. Hypertension should be avoided during all aortic cross-clamp manipulations, including the application and release of side-biting clamps.
5. Central venous pressure. With appropriate venous drainage, the CVP should be low (0 to 5 mm Hg). A persistently high CVP indicates poor venous drainage, which may require adjustment of the venous cannula or cannulas by the surgeon. Venous drainage can also be improved slightly by raising the operating table height, thereby increasing the hydrostatic gradient between the heart and the venous reservoir. Increasingly in recent years, suction (vacuum-assisted venous drainage) is applied to the venous reservoir, especially for miniaturized circuits (see Chapter 21), during which one should suspect excessive suction if the CVP reading should fall to levels below −5 mm Hg. As the CVP is a low-range pressure, it is very sensitive to measurement errors (e.g., hydrostatic gradient between transducer and right atrium). Care should also be taken to ensure that the catheter measuring the CVP is in a large central vein and is not snared by surgical tapes.
C. Fluid management and hemodilution
1. CPB Prime. The CPB circuit is “primed” with a balanced isotonic crystalloid solution, to which colloids, mannitol, or buffers may be added, depending on perfusionist, anesthesiologist, and surgical preference (see Chapter 21). CPB prime also contains a small dose of heparin (e.g., 5,000 to 10,000 units) and a dose of the antifibrinolytic agent being used (e.g., aminocaproic acid 5 g). The volume of the prime depends on the circuit components, but is typically about 800 to 1,200 mL for adults, and can be even lower when a miniaturized system is used (see Chapter 21).
2. Hemodilution. The use of a non-sanguineous prime inevitably results in hemodilution. The degree of hemodilution on commencement of CPB can be estimated prior to CPB by multiplying the Hb concentration (or Hct) prior to CPB by the ratio of the patient’s estimated blood volume to the patient’s estimated blood volume plus the CPB prime volume. Moderate hemodilution is usually well tolerated, because oxygen delivery remains adequate and oxygen requirements are often reduced during CPB, especially if hypothermia is used. Moderate hemodilution may also be beneficial, because it reduces blood viscosity, which counters the increase in blood viscosity induced by hypothermia.
6
3. Limits of hemodilution. While the safe limit of hemodilution during CPB in individual patients is not known, a conservative approach is to avoid Hb levels <6.5 g/dL (approximately an Hct of 20%). If the estimated degree of hemodilution on commencement of CPB is too low, allogeneic red blood cells (RBCs) can be added to the CPB prime. This is particularly important for smaller patients (due to their lower estimated blood volumes) (e.g., pediatric patients), and anemic patients. If venous oxygen saturations are low during CPB despite normal effective perfusion flow rates, excessive hemodilution as a cause should be considered, and additional RBCs added if necessary. Similarly, inadequate oxygen delivery will result in anaerobic metabolism and the development of acidosis. Patients with known stenoses of cerebral or renal arteries may be less tolerant of hemodilution.
4. Time course of hemodilution. During the course of CPB, crystalloid fluid will diffuse from the vascular to the extracellular space and also will be filtered by the kidney, gradually reducing the extent of hemodilution. However, crystalloid cardioplegia returning to the circulation will increase hemodilution, as will the addition of other crystalloids or colloids used to replace blood loss or redistribution of fluid into nonvascular compartments.
5. Monitoring hemodilution. The Hb (or Hct) should be measured frequently (e.g., every 30 to 60 min) (if possible, it should be monitored continuously), especially if there is ongoing blood loss, or low mixed venous oxygen saturations.
6. Acute normovolemic hemodilution. In adult patients with average (or greater) body size and normal preoperative Hb, acute normovolemic hemodilution prior to, or at the time of commencement of, CPB should be considered. Typically, 1 to 2 units of anticoagulated blood are collected, and replaced with colloids or a combination of crystalloids and colloids. This blood, containing pre-CPB Hb, platelet, and clotting factor levels can be re-infused post-CPB.
7. Allogeneic blood transfusion. The trigger for allogeneic RBC transfusion varies between institutions, and will depend also on patient and surgical factors. Conservative triggers are an Hb <6.5 g/dL during the maintenance phase of CPB, and <8.0 g/dL at the time of separation, although lower levels may be tolerated in selected patients.
8. Cardiotomy suction. Shed blood may be returned to the CPB circuit using cardiotomy suction. However, shed blood often contains activated coagulation and fibrinolytic factors, especially if exposed to the pericardium. Excessive cardiotomy suction may also be associated with hemolysis, especially if there is co-aspiration of air. For this reason, some choose to return only brisk blood loss to the CPB circuit. An alternative is separate cell salvage with washing of RBCs before returning them to the CPB circuit.
9. Fluid replacement. Fluid may be lost from the circuit through blood loss, redistribution to other compartments, and filtration by the kidney. A reduction in the circulating blood volume will manifest as a fall in the CPB reservoir fluid level. A falling CPB reservoir fluid level is dangerous, as it reduces the margin of safety for air embolism. In many circuits an alarm will be activated if the reservoir volume falls to unsafe levels. The replacement fluid is typically crystalloid with colloid added depending on perfusionist, surgeon, and anesthesiologist preference.
10. Diuresis and ultrafiltration. Occasionally the return of cardioplegia solution to the CPB circuit, or contraction of the vascular space by vasoconstrictors or hypothermia, will cause reservoir level to increase. If high levels persist, diuresis can be encouraged by the use of diuretic agents such as furosemide or mannitol. Alternatively, an ultrafiltration device can be added to the circuit to remove water and electrolytes (see Chapter 21).
11. Urine production should be identified and quantified as a sign of adequate renal perfusion and to assist in appropriate fluid management. Very high urine flow rates (e.g., >300 mL/h) may be seen during hemodilution (due to low plasma oncotic pressure), especially if mannitol is also present in the priming solution. Oliguria (less than 1 mL/kg/h) should prompt an investigation, because it may indicate inadequate renal perfusion. However, some hypothermic patients demonstrate oliguria without an apparent cause. Kinking of urinary drainage catheters should be excluded.
D. Management of anticoagulation (see also Chapter 19)
1. Monitoring anticoagulation. The ACT or a similar rapid test of anticoagulation must periodically confirm adequate anticoagulation (e.g., ACT > 400 s; see also Chapter 19). The ACT should optimally be checked after initiating CPB and every 30 min thereafter. The ACT can be checked within 2 min of administering heparin [6]. As the ACT falls over time, often a higher target is chosen (e.g., >500 s), so that the lowest ACT remains >400 s. During periods of normothermia, heparin elimination is faster, so a requirement for heparin supplementation is more likely.
2. Additional heparin is usually given in 5,000 to 10,000 unit increments, and the ACT is repeated to confirm an adequate response. Use of fully heparin-coated circuits does not eliminate the need for heparin; an ACT of 400 s or greater is often recommended [7].
3. Heparin resistance is a term used to describe the inability to achieve adequate heparinization despite conventional doses of heparin. It may be due to a variety of causes, but it is most common in patients who have received heparin therapy for several days preoperatively. Most cases will respond to increased doses of heparin. However, if an ACT > 400 s cannot be achieved despite heparin > 600 units/kg, consideration should be given to administering supplemental antithrombin III (AT-III). A dose of 1,000 units of AT-III concentrate will increase the AT-III level in an adult by about 30%. Fresh frozen plasma, 2 to 4 units, is a less expensive alternative, but it is less specific and carries the risk of infective and other complications. For a detailed discussion of heparin resistance and AT-III deficiency, see Chapter 19.
E. Temperature management
1. Benefits of hypothermia. Hypothermia during CPB reduces metabolic rate and oxygen requirements and provides organ protection against ischemia.
2. Disadvantages of hypothermia. Hypothermia may promote coagulation abnormalities, and may increase the risk of microbubble formation during rewarming. Hypothermia shifts the Hb oxygen saturation curve to the left, reducing peripheral oxygen delivery, but this is countered by the reduced oxygen requirements.
7
3. Choice of maintenance temperature. The optimal temperature during the maintenance phase of CPB is not known. Typically the patient’s core temperature at the onset of CPB is 35 to 36°C. Core temperature is usually measured in the nasopharynx or tympanic membrane, but the bladder or esophagus may also be used. The target temperature is chosen on the basis of the type and length of surgical procedure, patient factors, and surgical preference. Often the temperature is allowed to drift lower without active cooling. Alternatively, the heat exchanger is used to provide moderate hypothermia, which may be as low as 28°C, but is more often 32°C or above. If there is a concern about the adequacy of myocardial protection, lower temperatures may be used (see also Chapter 23).
4. Slow cooling. Lack of response of the nasopharyngeal or tympanic temperature during the cooling phase may indicate inadequate brain cooling, and should prompt investigation of the cause (e.g., ineffective heat, exchanger, inadequate cerebral perfusion). The position and function of the temperature monitor should also be checked to exclude artifactual causes.
5. Deep hypothermic circulatory arrest (DHCA). For certain surgical procedures in which circulatory arrest is required (e.g., repairs of the aortic arch), deep hypothermia is used as part of a strategy to prevent cerebral injury. The typical target temperature prior to circulatory arrest is about 15 to 17°C. Other strategies to minimize injury include limiting the period of circulatory arrest to as short a time as possible, anterograde or retrograde cerebral perfusion during the period of DHCA, and pharmacological protection using barbiturates (e.g., thiopental 10 mg/kg), corticosteroids (e.g., methylprednisolone 30 mg/kg), and mannitol (0.25 to 0.5 g/kg). These must be given before DHCA is commenced (see also Chapter 25). Achieving deep neuromuscular blockade (0–1 twitches on train-of-four) prior to DHCA is advisable.
6. Rewarming. Rewarming commences early enough to ensure that the patient’s core temperature has returned to 37°C by the time the surgical procedure is completed, so that separation from CPB is not delayed. The surgeon will usually advise the perfusionist when rewarming should commence, taking into account the patient’s core temperature at the time, how long the patient has been at this temperature, and the patient’s body size. The rate of rewarming is limited by the maximum safe temperature gradient between the water temperature in the heat exchanger and the blood (<10°C, some centers use a maximum of 6 to 8°C). Higher gradients risk the formation of microbubbles. Typically, patients’ core temperature rises no faster than 0.3°C/min. Vasodilators may facilitate rewarming by improving distribution of blood and permitting higher pump flow rates.
7. Hypothermia and arterial blood gas analysis. Hypothermia increases the solubility of oxygen and carbon dioxide, thereby reducing their partial pressures. However, arterial blood gas measurement is performed at 37°C, so the values have to be “temperature corrected” to the patient’s blood temperature if the values at the patient’s blood temperature are required. The reduced PaO2 is of limited clinical significance, so long as increased fractions of oxygen are administered (FiO2 > 0.5). However, the reduced PaCO2 produces an apparent respiratory alkalosis when temperature-corrected values are used. To keep the pH normal (pH stat) it would be necessary to add CO2 to the oxygenator. The alternative is to avoid temperature correction of arterial blood gases and accept that the degree of dissociation of H+ also varies with temperature (alpha stat). With this strategy there is no requirement to add CO2 to maintain neutrality. These complex biochemical considerations are avoided by using non–temperature-corrected values, and making decisions based on the values measured at 37°C, irrespective of the patient’s blood temperature. See Chapter 24 for detailed discussion of arterial blood gas management.
8. Shivering. Shivering should not occur if adequate anesthesia is provided, especially if a muscle relaxant is administered.
F. ECG management. Isolated atrial and ventricular ectopic beats are common during cardiac manipulation and require no specific intervention. If ventricular fibrillation occurs before aortic cross-clamp placement, defibrillation may be required. Ventricular fibrillation once the aortic cross-clamp has been placed is likely to be short-lived because the delivery of cardioplegia will achieve cardiac standstill. Persistent ventricular fibrillation indicates ineffective cardioplegia. Return of electrical activity after cardioplegic arrest suggests washout of cardioplegia solution. The surgeon should be notified as additional cardioplegia may be required. Ventricular fibrillation may occur during the rewarming phase after the release of the aortic cross-clamp. This often resolves spontaneously, but may require defibrillation, especially if the patient remains hypothermic.
G. Myocardial protection (see also Chapter 23)
1. Cardioplegia. When the myocardial blood supply is interrupted by the placement of an aortic cross-clamp, cardioplegic arrest of the myocardium is required. The antegrade technique is achieved by administering cardioplegia solution into the aortic root between the aortic valve and aortic clamp. The interval between the placement of the cross-clamp and the administration of the cardioplegia is kept to a minimum (no more than a few seconds) to prevent any warm ischemia. The cardioplegia solution is typically high in potassium, arresting the heart in diastole. The solution is typically cold (8 to 12°C) to provide further protection, although warm continuous cardioplegic techniques are used in some institutions. Cardioplegic solutions may be entirely crystalloid or may be mixed with blood (blood cardioplegia). Cardioplegia may also be administered retrograde through a catheter in the coronary sinus. In patients with aortic regurgitation, administration of cardioplegia directly into the left and right coronary ostia may be required. Cardioplegia is typically given intermittently every 20 to 30 min, but may be given continuously.
8
2. Cold. Most myocardial protection techniques involve cold cardioplegia, and ice may be placed around the heart to provide further protection. Systemic hypothermia, if used, contributes to keeping the myocardium cold.
3. Venting. During cross-clamping, vents are typically placed in the aortic root to ensure that the heart does not distend. For open-chamber procedures vents are placed also in the left atrium or LV to remove both blood and air. Inadequate venting may result in the development of tension in the LV, causing potential ischemia and subendocardial necrosis. The coronary perfusion pressure for cardioplegia is also reduced.
4. Avoiding electrical activity. See Section IV.F above.
H. Arterial blood gas and acid–base management
1. Alpha stat or pH stat strategy? (see Section IV.E.7 and Chapter 24)
2. The arterial PO2 is maintained between 150 and 300 mm Hg by adjusting the percentage oxygen in the sweep (analogous to inspired) gas delivered to the oxygenator. Arterial hypoxemia may indicate inadequate oxygenator sweep gas flow (or leak) or inadequate oxygen percentage in the oxygenator sweep gas. Alternatively, it may indicate oxygenator dysfunction.
3. The arterial Pco2 is maintained at approximately 40 mm Hg by adjusting the sweep gas flow rate through the oxygenator. There is an inverse relationship between the sweep gas flow rate and the arterial PCO2. Hypercapnea (PCO2 > 45 mm Hg) should be avoided as it is associated with sympathetic stimulation and respiratory acidosis. Hypercapnea may be caused by an inadequate sweep gas flow rate, absorption of CO2 used to flood the wound during open chamber procedures [8], or increased CO2 production. The administration of bicarbonate also increases the PCO2. Hypocapnea (PCO2 < 35 mm Hg) should also be avoided as it is associated with respiratory alkalosis and left shift of the HbO2 dissociation curve (further reducing oxygen delivery), and cerebral vasoconstriction.
4. Metabolic acidosis (e.g., lactic acidosis) is prevented where possible by ensuring adequate oxygen delivery and tissue perfusion. Severe metabolic acidosis should be corrected cautiously with the use of sodium bicarbonate. If unexplained acidosis occurs with signs of an increased metabolic rate (e.g., low mixed venous oxygen saturations, elevated PCO2), malignant hyperthermia (MH) should be considered.
I. Management of serum potassium and sodium
1. Hyperkalemia may occur when cardioplegia solution (which contains high potassium concentrations) enters the circulation. This is usually mild or transient unless large amounts of cardioplegia are used, or the patient has renal dysfunction. Hyperkalemia more often follows the first dose of hyperkalemia than later ones, because both the volume and potassium concentration are typically higher for the initial cardioplegia solution. Hyperkalemia can cause heart block, negative inotropy, and arrhythmias. Hyperkalemia can be treated by promoting potassium elimination by loop diuretics (e.g., furosemide) or by ultrafiltration. Potassium can also be shifted into cells by the administration of insulin and glucose, or by creating an alkalosis. In rare cases, hemodialysis is required. If the patient has severe renal dysfunction or the serum potassium remains above the normal range, the cardioplegia delivery technique should be modified to ensure that cardioplegia is vented separately and not returned to the circulation.
2. Hypokalemia. If a patient is hypokalemic, initiating K+ replacement during CPB is much safer than waiting until after bypass, thus avoiding hypokalemic dysrhythmias during CPB weaning or potential cardiac arrest during rapid K+ replacement post-CPB.
3. Sodium. Serum sodium should be maintained within the normal range where possible. Rapid corrections should be avoided due to the risk of acute changes in intracranial pressure as a result of the changes in plasma osmolality.
J. Management of blood glucose
1. Hyperglycemia. Glucose tolerance is often impaired during CPB due to the stress response associated with CPB, as well as from insulin resistance induced by hypothermia. Hyperglycemia may exacerbate neuronal injury and increase the risk of wound infection. Blood glucose should be measured frequently, especially in patients with diabetes mellitus. Glucose containing fluids should be avoided. Blood glucose should optimally be maintained below 180 mg/dL, which may require the infusion of insulin.
2. Hypoglycemia. Hypoglycemia should be avoided at all costs during CPB, because severe hypoglycemia is associated with neurological injury within a short period, and the signs of hypoglycemia are masked by both the anesthesia and the hemodynamic changes during CPB. Blood glucose should be measured more frequently if patients are receiving insulin or have received hypoglycemic agents preoperatively on the day of surgery.
V. Rewarming, aortic cross-clamp release, and preparation for weaning
A. Rewarming. On commencement of rewarming, additional anesthetics may be required, because the brain rewarms faster then the body core. Additional heparin may be required, because the rate of metabolism of heparin returns to normal at normothermia. The extent of hemodilution should be re-assessed because oxygen requirements increase during rewarming (see also Section IV.E.6 above).
B. Release of aortic cross-clamp
1. De-airing. Air may collect in the pulmonary veins, left atrium, or LV, particularly during open chamber procedures. This is aspirated through the aortic root vent prior to cross-clamp release or other vents. Temporarily raising the CVP and inflating the lungs will fill the LV and permit easier surgical aspiration of intracavity air. Residual air can be detected using TEE [9]. Flushing the surgical field with CO2 prior to cardiac chamber closure [8] may reduce residual air, as CO2 is reabsorbed much faster than air.
2. Blood pressure. Hypertension should be avoided at the time of aortic cross-clamp release. Transient hypotension may occur after the release of the cross-clamp due to residual cardioplegia or metabolites returning to the circulation as the myocardium is reperfused.
C. Preparation for weaning from CPB. In preparation for weaning from CPB, cardiac pacing equipment is attached and checked, electrolytes and acid–base disturbances are corrected if necessary, an adequate Hb is ensured, and additional inotropic drug infusions (e.g., epinephrine, dobutamine) required for the weaning process are prepared and attached to the patient. If loading doses of inodilators (e.g., milrinone) or calcium sensitizers (e.g., levosimendan) are required, these should be given before completion of CPB. If the negative inotropic effects of volatile agents are a concern, they should be ceased before weaning commences, and other agents used to maintain adequate anesthesia. Anesthetic management of weaning from CPB is covered in Chapter 9.
VI. Organ protection during CPB
A. Renal protection. The most important renal protective strategy is to ensure adequate renal perfusion during CPB by optimal fluid loading, appropriate pump flow rates, close attention to the renal perfusion pressure, and avoidance of intravascular hemolysis and hemoglobinuria. It may be possible to reduce the risk of development of acute renal failure through the use of drugs to increase renal blood flow and urine production, although there is no definitive evidence to support their routine use. Mannitol, low-dose dopamine, furosemide, prostaglandin E, and fenoldopam (a selective dopamine-1 receptor agonist) have been advocated for use in high-risk patients during CPB, particularly if oliguria is present. Of these, fenoldopam 0.05 to 0.10 μg/kg/min shows the most promise [10]. N-acetylcysteine (a free-radical scavenger) and urinary alkalinization have also been used. Hemolysis and hemoglobinuria are managed by correcting the cause where possible, and by promoting a diuresis.
B. Brain protection during CPB involves ensuring adequate cerebral perfusion pressure (MAP-CVP) and oxygen delivery, and measures to prevent increases in intracranial pressure (which will reduce cerebral perfusion pressure). Mild or moderate hypothermia is often used to provide additional protection, and deep hypothermia if circulatory arrest is required (see also Section IV.E.5 above). Care is taken to avoid emboli, both particulate (e.g., atheroma) and gaseous, by meticulous surgical and perfusion technique. Brain protection is covered in detail in Chapter 24.
C. Myocardial protection. See Section IV.G above.
D. Inflammatory response to CPB. CPB is one of the main factors contributing to the inflammatory response associated with cardiac surgery. Reactions are usually mild or subclinical, but may be severe in some cases and contribute to brain, lung, renal, or myocardial injury. For a detailed discussion of this inflammatory response to CPB and cardiac surgery, see Chapter 21.
1. Etiology
a. Exposure of blood to circuit components. The extensive contact between circulating blood and the extracorporeal circuit results in variable amounts of thrombin generation, activation of complement, release of cytokines, and expression of immune mediators, all of which may contribute to the inflammatory response.
b. Return of shed blood to the CPB circuit. Shed blood is in contact with mediastinal tissues (e.g., pericardium) and air, and is exposed to shear stress when suction is used. It is a potent source of activated coagulation factors and inflammatory mediators and may cause hypotension when returned to the bypass circuit. Unless bleeding is brisk or stasis is minimal, shed blood should not be returned directly to the CPB circuit. A cell saver can be used to conserve red blood cells.
c. Ischemia due to inadequate tissue perfusion or organ protection
d. Endotoxemia due to splanchnic hypoperfusion
2. Prevention
Severe reactions are difficult to predict or prevent. Adequate anticoagulation, organ perfusion, and myocardial protection are fundamental. Biocompatible surface coated circuits may be beneficial. The use of miniature bypass circuits, steroids, and leuco-depletion filters are controversial. Fibrinolysis can be reduced by the use of aminocaproic or tranexamic acid. Novel anti-inflammatory agents (e.g., pexelizumab) [11] remain investigational, but as yet have no proven benefit.
3. Management
Low SVR and evidence of capillary leak may be observed during CPB, but most reactions manifest post-CPB. No specific therapy is available and management is supportive.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








