TOPICS
1. Diseases of the ascending thoracic aorta
2. Diseases of the descending thoracic aorta
3. Anesthetic management of the patient with disease of the thoracic aorta
4. Distal aortic perfusion and spinal cord protection during descending thoracic aneurysm resection
5. Deep hypothermic circulatory arrest
6. Echocardiography in the management of thoracic aortic diseases
With each heartbeat blood is ejected into the aorta exerting multiple mechanical forces on it: pressure, radial and longitudinal stress, tension. Just as diseases of the aorta can affect the heart, diseases of the heart can affect the aorta. Cardiac patients often suffer from both and this makes their clinical care more complex.
The aorta ascends in the anterior mediastinum, curves backward into the aortic arch from which emanate the great vessels of the head and the upper extremities, descends into the posterior mediastinum and beyond the diaphragm continues into the abdomen providing blood to the spinal cord, gut, kidneys, ultimately dividing to deliver blood to the lower extremities. Diseases that interfere with the delivery of blood to the tissues (eg, aortic dissections, atherosclerosis, and emboli) place patients at great risk for organ ischemia. Other disease conditions (eg, aneurysms) weaken the wall of the aorta and often result in aortic rupture and sudden death. Many patients with aortic disease present emergently in the setting of acute aortic dissection, contained aneurysmal rupture, or following blunt or penetrating traumatic aortic injury. Others, with long-standing aortic aneurysms, present for elective surgery for repair of progressive dilatation of the thoracic aorta. Irrespective of the nature of their thoracic aorta disease these patients are often quite unstable and their prognosis can be disappointing. Consequently, cardiac surgeons and their anesthesia colleagues are called upon to exert great skill to manage these “high-risk” patients.
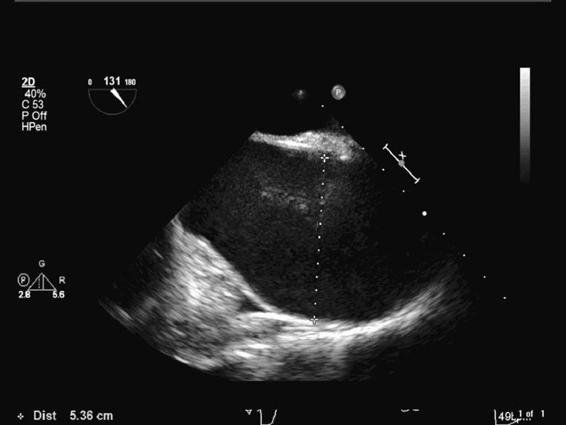
Figure 9–1. This long-axis TEE view of the ascending aorta demonstrates a 5.36 cm dilatation.
DISEASES OF THE ASCENDING THORACIC AORTA
Patients with ascending thoracic aortic aneurysms present either acutely or electively (Figure 9–1). Crushing chest pain often heralds acute presentations. Suffice it to say these patients may never undergo surgery as they bleed and/or tamponade to death. However, sometimes ruptures are contained such that patients do not exsanguinate or tamponade. In these cases, the patient will present emergently for repair following diagnosis in the ER. Acute, contained ascending aortic aneurysm ruptures require immediate surgical correction. Radiographic (MRI, CT) and ultrasound techniques are routinely employed to make the diagnosis of a thoracic aortic aneurysm. Rapid accumulation of blood in the pericardium results in a tamponade physiology necessitating emergency surgical treatment.
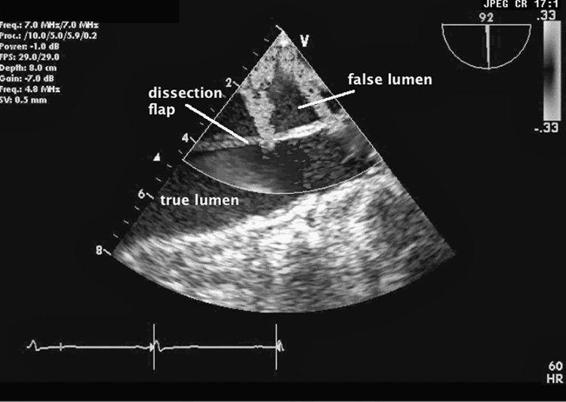
Nonemergent presentations occur when patients develop aneurismal dilatations over time and become symptomatic from anatomic compression of the trachea, the main bronchi, or the esophagus by the expanding aneurysm. At times, an ascending aneurysm widens the aortic root making the aortic valve leaflets no longer competent leading to severe aortic insufficiency (AI). These patients often present with the symptoms of aortic regurgitation. Echocardiography will readily demonstrate dilatation of the aortic root and aortic valvular incompetence. Often a widened mediastinum on routine x-ray examination may herald the presence of an ascending thoracic aneurysm. Chronic aneurismal dilatations are regularly followed with surgical repair suggested either when the patient becomes symptomatic or when the aneurysm becomes greater than 5 to 6 cm in diameter. Ascending aortic aneurysms are associated with Marfan syndrome, atherosclerosis, aortic stenosis, and infectious aortitis. Aortic dissections occur when a tear in the intimal layer of the aorta’s wall permits blood under arterial pressure to create a false lumen in the wall of the aorta (Figure 9–2 and Videos 9–1 and 9–2). This false lumen can propagate along the entire length of the aorta. As the dissection expands, the false lumen compresses the true lumen and prevents blood from flowing down the aorta’s many branches including the coronary, carotid, subclavian, spinal, mesenteric, and renal arteries. Consequently, an aortic dissection can readily result in severe end-organ ischemia producing myocardial infarction, stroke, renal failure, and visceral ischemia leading to dead bowel and sepsis. The aortic root becomes distorted resulting in aortic valve incompetence and acute aortic insufficiency. Aortic dissections can also result in bleeding into the pericardium resulting in tamponade.
Figure 9–2. An aortic dissection is seen using color Doppler echocardiography. The true lumen (TL) and the false lumen (FL) are separated by the dissection flap. There are two color jets reflecting blood flow proceeding from the TL into the FL.
There are two available classifications of aortic dissections.
The DeBakey classification consists of three different types:
Type I: The intimal tear occurs in the ascending aorta but the dissection extends all the way to the descending aorta.
Type II: The intimal tear occurs in the ascending aorta and the dissection is limited to the ascending aorta.
Type III: The intimal tear occurs distal to the take off of the left subclavian artery with the dissection extending into the descending aorta for variable distances.1
The Stanford (Daily) identifies two types of dissections:
Type A dissections have involvement of the ascending aorta regardless of the location of the intimal tear or of the extension of the dissection into the descending aorta.
Type B dissections involve only the descending aorta distal to the take off of the left subclavian artery.2
While type B dissections are usually managed conservatively unless organ hypoperfusion, hemodynamic instability or persistent pain are present, type A dissections represent a true surgical emergency.
Surgeons approach the ascending aorta through a median sternotomy much as they would do for a routine cardiac case. If the aortic arch and the great vessels to the head are not involved, the surgeon places an aortic cross clamp distal to the location of the aortic pathology and resects the diseased aorta using conventional cardiopulmonary bypass (CPB). Depending on the degree of aortic annulus dilatation and the severity of the aortic insufficiency the aortic valve is either replaced or not replaced. A composite graft can be sewn between the left ventricular outflow tract and the patient’s native aorta. Since the right and left coronary arteries branch from the ascending aorta they must be separated from the aorta and then sewn back into the new ascending aortic graft.
Should the aortic pathology involve the entire ascending aorta, the intraoperative management of the patient is more complicated. Recall from Chapter 4 that during a routine cardiac surgery case with CPB the surgeon generally places the aortic perfusion cannula in the ascending thoracic aorta. An aortic cross clamp is placed below the perfusion cannula to isolate the heart from the systemic circulation. The coronary arteries at this point are no longer perfused and cardioplegia solution is administered via an anterograde cardioplegia cannula.
Therefore, if the aortic pathology involves the entire ascending aorta, the femoral artery or the right axillary artery are typically cannulated. The cross clamp is placed beyond the diseased aorta. Cardioplegia is delivered in the aortic root or if significant aortic insufficiency is present, directly into the left and right coronary ostia after aortotomy. Alternatively, retrograde cardioplegia can be administered in addition or in lieu of antegrade cardioplegia. Once a new aortic graft is sewn into place, the native coronary arteries are attached to the new aortic graft, if necessary. When surgery is completed the heart is de-aired, the cross clamp released, blood flows down the reattached right and left coronary arteries, and the patient hopefully is successfully weaned from CPB.
Unfortunately, this routine management scheme is not applicable when the disease involves the aortic arch and the innominate artery, left subclavian and left carotid arteries are involved. In these instances, aortic blood flow to these vessels will be interrupted while the aortic arch is reconstructed. In order for the surgery to proceed, deep hypothermic circulatory arrest is used and selective retrograde (via the superior vena cava) or antegrade (via the right axillary artery) perfusion of the head vessels may be used (see following discussion).
DISEASES OF THE DESCENDING THORACIC AORTA
Descending thoracic aortic aneurysms occur distal to the take off of the left subclavian artery from the aortic arch. While cardiac surgeons repair ascending aneurysms, both vascular and thoracic surgeons may be involved in the repair of descending thoracic aneurysms. Aneurysms of the descending thoracic aorta are usually secondary to atherosclerosis and these patients frequently have multiple other vascular diseases (eg, carotid occlusion, coronary artery disease). As with ascending thoracic aneurysms, presentation can be secondary to acute rupture with contained bleeding or detected on routine examination. Rupture without tissue containment of bleeding rapidly would lead to exsanguination and death.
The surgical approach to the descending thoracic aorta is via a left thoracotomy. Once the aorta is dissected free from surrounding tissues, cross clamps are placed above and below the lesion. The aneurysm is resected and a tube graft sewn into position. More recently, endovascular stents have been employed by surgeons and radiologists to exclude the aneurysmal walls from the circulation.3 Using radiological guidance, a stent is positioned in the aorta such that blood flows through the aortic stent, therefore arterial pressure does not contact the diseased aortic wall. Stents require areas of relatively normal aorta at their proximal and distal ends. Additionally, when the stent is deployed, any artery, which takes off from the aorta in the stented area, will no longer receive aortic blood flow because the stent occludes it. Consequently, perfusion of the spinal cord, viscera, and kidneys may be impaired.
The placement of aortic cross clamps often leads to significant hemodynamic changes with the development of hypertension above the proximal clamp and profound hypotension below the distal cross clamp. Insufficient arterial blood flow to the kidneys, viscera, and spinal cord can occur resulting in organ ischemia.
When performing descending thoracic artery surgery and using the simple cross-clamp technique, the surgeons attempt to complete their procedure as rapidly as possible in order to minimize the time of distal aortic hypoperfusion. Various mechanisms of distal aortic perfusion and spinal cord protection (see below) have been advocated to mitigate injuries associated with distal aortic hypoperfusion and the occlusion of branching arteries.
ANESTHETIC MANAGEMENT OF THE PATIENT WITH DISEASE OF THE THORACIC AORTA
Anesthetic considerations in the management of the patient with surgical thoracic aortic disease take into consideration the location of the aortic pathology and the elective or emergent nature of the surgery.
Patients presenting with an isolated ascending aneurysm are usually scheduled for elective resection and are often managed in a manner similar to routine cardiac surgical patients as described in Chapter 4. Arterial line placement in the right radial artery can be useful to detect unwanted innominate artery occlusion by the aortic cross clamp. Hypertension should be carefully avoided during the peri-induction period as the aortic walls of the aneurysm are fragile and one would not want to rupture the aneurysm on induction. Surgery usually involves excision of the diseased ascending aorta and interposition of a synthetic graft between the aortic valve and the proximal aortic arch. A rarely used repair without synthetic graft involves partial excision of some of the aortic wall through a diagonal aortotomy and suturing the vessel to a smaller size. Coronary ostia may need to be excised and reimplanted into the graft. When significant dilatation of the aortic annulus and aortic insufficiency is present, the aortic valve is also replaced together with the ascending aorta using a composite graft. Patients are weaned from CPB in the usual fashion if standard cannulation and bypass techniques are used.
Patients presenting with acute ascending aortic dissections or contained aortic ruptures will present with a variety of pathologies. Documentation of baseline neurological function is necessary since some patients may have neurological injury secondary to inadequate perfusion of the carotid arteries preoperatively. Other patients with acute dissections or ruptures may present with myocardial ischemia due to involvement of the coronary ostia, tamponade, and/or acute aortic regurgitation. Such patients may be in heart failure upon their arrival in the operating room. These patients often will tolerate limited amounts of anesthetics (intravenous or otherwise) and their management prior to the initiation of CPB is largely resuscitative in nature. Unstable cardiac patients are at risk for intraoperative awareness and bispectral index monitoring is suggested though it may have limited benefit. Older drugs like scopolamine may be useful in these situations to attenuate recall.
Should aortic pathology extend into the aortic arch, deep hypothermic circulatory arrest (DHCA) is employed to halt the circulation while the aortic arch is being repaired. This is necessitated by the inability to cannulate and cross clamp in the ascending aorta. A cannula is placed in the femoral artery or in the right axillary artery in order to deliver arterial blood from the bypass machine into the systemic circulation.
During DHCA the patient’s blood is cooled by the bypass machine and core temperature is reduced to less than 18°C. The patient’s head is packed in ice bags. Adequate cooling of the brain can be confirmed by nasopharyngeal temperature monitoring or more accurately by electroencephalography (EEG) to ensure that isoelectric EEG has been reached prior to instituting DHCA. The bypass machine is turned off such that there is no flow from the machine back to the patient. Deep hypothermia reduces the risk of ischemic injury and the surgeon attempts to replace the aorta with an aortic graft as quickly as possible. Following resection of the aneurysm, the distal anastomosis of the graft is performed. The vessels of aortic arch are reattached to the tube graft as quickly as possible. The graft can be clamped now proximal to the innominate artery and the flow to the descending aorta, arch and head reestablished by reinstituting CPB. Next, the proximal graft anatomosis is completed, flow is restored to the coronary arteries, and, after a long rewarming period, weaning from CPB is accomplished hopefully without any ischemic injury.
Patients with aortic dissections distal to the left subclavian artery have distal thoracic or thoracoabdominal disease and are often managed medically. In surgical cases, anesthetic induction as with all cardiac surgery patients must be individualized based upon each patient’s own hemodynamic behavior. A mixture of amnestics, analgesics, hypnotics, and muscle relaxants can generally be employed. However, at times patients undergoing descending thoracic aneurysm surgery will be monitored with sensory and/or motor evoked potentials. Because spinal cord perfusion can be compromised during the cross-clamp period secondary to occlusion of the radicular arteries, surgeons attempt to detected spinal cord ischemia through the use of neurophysiological monitoring. Since muscle relaxants and inhalational agents interfere with these monitors, total intravenous anesthesia is at times required in these cases.
Descending thoracic aneurysm resections also require one-lung ventilation to improve surgical exposure. Either double-lumen endotracheal tubes or bronchial blockers can be employed to achieve isolation of the left lung. Bronchial blocker techniques may have the advantage of leaving a single lumen tube in place at the end of surgery. Since these are rather protracted cases on rather sick individuals, extubation at the immediate conclusion of surgery is not recommended. Double-lumen endotracheal tubes generally need to be changed to single-lumen tubes prior to the patient’s transfer to the ICU. Unfortunately, many patients following descending thoracic aneurysm surgery frequently become edematous from perioperative fluid administration and head down position. Consequently, re-intubation can be challenging making use of the bronchial blocker a potentially more facile way of managing one-lung ventilation perioperatively.
After discussing the anesthetic and surgical plan with the surgical team, a lumbar cerebrospinal fluid drain is often placed when performing distal thoracic aneurysm resections. During cross clamp of the distal aorta, blood flow to the spinal cord is reduced secondary to decreased perfusion of the radicular arteries. Thus, the spinal cord is at risk for ischemia. Removing cerebrospinal fluid is thought to improve spinal cord perfusion pressure by lowering CSF pressure. Before placing the lumbar drain the patient’s coagulation status and medications should be confirmed and strict sterile technique must be employed.
DISTAL AORTIC PERFUSION AND SPINAL CORD PROTECTION DURING DESCENDING THORACIC ANEURYSM RESECTION
Much of what is unique in the management of thoracic aortic aneurysm patient is centered upon reducing or preventing ischemic injury to the tissues secondary to inadequate perfusion during repair of the aorta.
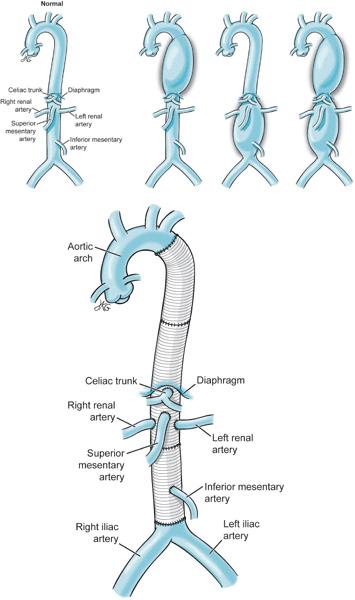
Descending thoracic aortic aneurysms were originally performed with a “clamp and go” (Figure 9–3) technique.4 In this approach, following cross clamping of the aorta the surgeon would simply attempt to sew in the aortic graft as quickly as possible. By minimizing ischemic time, injuries to the kidneys, viscera, and spinal cord hopefully might be prevented. Cross clamping the aorta produces systemic hypertension above the proximal clamp and profound hypotension below the distal clamp. Surgeons use various techniques to minimize the impact of cross clamping on hypoperfused organs including: CSF drainage, systemic patient cooling, distal aortic perfusion, and intercostal artery implantation. Monitoring of the evoked potentials is also employed to alert surgeons of spinal cord ischemia. CSF is drained to improve spinal cord perfusion pressure by keeping the CSF pressure to less than 10 mm Hg.5 However, if CSF is drained too quickly, intracranial hypotension can develop which can lead to cerebral vein tearing and intracranial hemorrhage. Individual protocols may limit the total amount of CSF drained (eg, < 240 mL/d) to minimize this risk.6 CSF catheter drainage may also place patients at risk for perioperative meningitis as the catheter remains in place for some time postoperatively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree