FIGURE 9.1 Sagittal image of a middle-aged woman who has a 4 cm × 4 cm mass in the cerebellopontine angle.
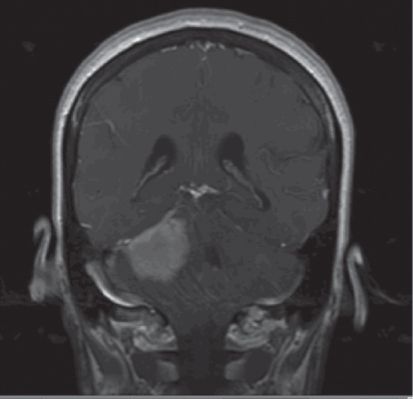
FIGURE 9.2 Coronal image of a middle-aged woman who has a 4 cm × 4 cm mass in the cerebellopontine angle.
This presentation is consistent with an acoustic neuroma, without associated neurofibromatosis Type II. Preparation for this case can be aided by review of the Six Questions for Posterior Fossa Surgery (Fig. 9.3).

FIGURE 9.3 Six Questions For Posterior Fossa Surgery. Six questions to consider before caring for patients undergoing posterior fossa surgery.
1. Does the patient have preoperative evidence of brain stem or cranial nerve dysfunction? Yes. This patient has evidence of sensorineural hearing loss and dizziness as a result of the disturbance of vestibular–cochlear function in Cranial Nerve VIII. The occasional facial twitching is an indication that the tumor is causing local compression of Cranial Nerve VII.
2. Does the patient have elevated intracranial pressure (ICP)? She has evidence of obliteration of the fourth ventricle on imaging but is not exhibiting clinical symptoms of hydrocephalus or elevated ICP.
3. What is the proposed patient position for operation? Supine, with the head turned to the left.
4. Is the patient at risk for venous air embolus (VAE)? Moderate risk. The risk for VAE in the horizontal patient undergoing posterior fossa surgery is approximately 15% (range: 0% to 17%). The time of increased risk is during craniectomy when non-compressible skull veins are exposed and during dissection near the sinuses.
5. Is there potential for large blood loss? Low-to-moderate risk. The location and the characteristics of the tumor do not suggest large blood loss. One must consider the involvement of sinuses, vascularity of the mass, and the skill of the surgeon when assessing this component of the case.
6. Will the surgery involve intraoperative central nervous system (CNS) monitoring? Yes. Electromyographic (EMG) monitoring of Cranial Nerves V and VII is often used to help identify structures and to minimize facial weakness resulting from dissection near these delicate structures. For large lesions at the cerebellopontine angle threatening the lower cranial nerves, Cranial Nerve X may also be monitored by EMG. Brain stem auditory evoked response (BAER) monitoring of Cranial Nerve VIII may be used to help preserve residual hearing.
CHAPTER HIGHLIGHTS
This chapter reviews the anesthetic considerations for posterior fossa surgery. Key points include the following:
 Venous air embolus (VAE)
Venous air embolus (VAE)
 Intraoperative monitoring techniques
Intraoperative monitoring techniques
 Positioning
Positioning
 Pathophysiology associated with brain stem dysfunction
Pathophysiology associated with brain stem dysfunction
ANESTHESIA FOR POSTERIOR FOSSA SURGERY
Operations in the posterior fossa are often demanding, delicate, and long. Anesthesia for these cases can also be challenging. In addition to the anesthetic management issues that apply to supratentorial surgery, operations in the posterior fossa present special problems related to brain stem dysfunction, patient positioning, intraoperative neurophysiologic monitoring, and the potential for VAE.
I. ANATOMICAL CONSIDERATIONS
A. The posterior fossa is a small, rigid compartment containing the midbrain pons, medulla, cerebellum, and fourth ventricle. Surgery within the posterior fossa may be indicated for resection of vascular lesions or tumors, decompression of cranial nerves, and correction of craniocervical abnormalities. The common goal of the surgeon and the anesthesiologist is to facilitate safe exposure with minimal retraction and consequent tissue damage and edema.
B. The reticular activating system, cranial nerves, and structures vital for control of the airway and cardiovascular and respiratory systems are contained within a very small space in the posterior fossa. Accordingly, patients may present with dysphagia, laryngeal dysfunction, respiratory irregularities, cardiac rhythm disturbances, or altered states of consciousness. In some cases, chronic aspiration owing to loss of airway reflexes can further compromise respiratory function. These abnormalities may be encountered intraoperatively, associated with traction or nerve manipulation, and postoperatively as a result of mechanical trauma, edema, or bleeding in this confined space.
C. Hydrocephalus from obstruction of ventricular outflow is a common cause of increased ICP with posterior fossa lesions. This is evident on the preoperative computed tomographic (CT) or magnetic resonance imaging (MRI) scan as obliteration of the fourth ventricle or loss of the cerebellar folds. It can be treated by ventricular drainage, endoscopic third ventriculostomy, or hypertonic osmotherapy with mannitol and furosemide administered either pre- or intraoperatively.
II. POSITIONING
A. General issues. Posterior fossa surgery often requires unusual patient positioning. The prone, lateral, park bench, and sitting positions are commonly used. Regardless of the position chosen, care in positioning is of utmost importance because most problems are presumably avoidable with careful positioning and padding of vulnerable areas.
1. In the prone position, facial skin ulcerations can occur from uneven pressure distribution when head rests are used, and blindness can result from pressure on the globe of the eye. Ischemic optic neuropathy is associated with prolonged procedures in the prone position even when direct pressure on the eye is absent. Risk factors associated with this condition include significant blood loss, anemia, and hypotension.
2. In the lateral and the park bench positions, there is a risk of brachial plexus injury if the up arm is pulled caudally to gain access to the retromastoid area. There can also be nerve compression on the dependent side from pressure on the axillary contents or the peroneal nerve at the knee.
3. Excessive neck rotation can also stretch and damage the brachial plexus. Extreme neck flexion is associated with the risk of quadriplegia, especially in the sitting position (see below). As this can be exacerbated by hypotension, measurement of the blood pressure (BP) at the level of the cervical spine is recommended.
B. The sitting position
1. Advantages of the sitting position include good surgical exposure, improved ventilation, better access to the airway, greater comfort for the surgeon, and possibly reduced blood loss.
2. Disadvantages of the sitting position include the risk of VAE, increased chance of pneumocephalus, and the potential for hemodynamic instability. Additional complications include sciatic nerve injury from extreme flexion of the hip, massive swelling of the face and tongue from extreme neck flexion and/or rotation, and midcervical quadriplegia. The cause has been attributed ostensibly to a combination of stretch and compression of the spinal cord by extreme neck flexion in conjunction with hypotension.
3. The main contraindication to the use of the sitting position is the presence of a documented right-to-left intracardiac or pulmonary shunt, which would facilitate systemic embolization of air.
4. When caring for patients in the sitting position, BP is measured at the level of the head. BP measured at the level of the heart will underestimate the perfusion pressure for the brain by about 2 mm Hg for every inch the head is above the heart.
III. VENOUS AIR EMBOLISM
A. VAE can occur whenever pressure within an open vessel is subatmospheric. This situation is more likely to be encountered when the surgical site is above the level of the heart. Hence, VAE is a particular problem during surgery in the seated position, but it also occurs, albeit less frequently, in patients operated in the lateral or the prone position.
B. When open vessels cannot collapse, which is the case with major venous sinuses as well as bridging and epidural veins, the risk of VAE increases substantially. Most studies indicate that the incidence of VAE during posterior fossa procedures in the sitting position is 40% to 45% (range: 0% to 70%). For seated cervical laminectomy or operations in the prone or the lateral position, VAE occurs in approximately 10% to 15% of cases.
C. VAE can occur in varying amounts and duration with varying effects.
1. Massive air embolism produces abrupt and catastrophic hemodynamic changes. Fortunately, this type of VAE is rare.
2. More commonly, air entrainment occurs slowly over a longer period of time. This may produce little or no respiratory or hemodynamic compromise. However, it may result in increased right-sided cardiac pressures, increased dead-space ventilation, and hypoxemia.
a. As air is cleared to the pulmonary circulation, pulmonary vascular resistance and pulmonary artery (PA) and right atrial pressures increase. If unchecked, cardiac output can decrease as a result of right-heart failure and/or reduced left ventricular filling.
b. This vascular obstruction increases dead-space ventilation, resulting in a decrease in end-tidal carbon dioxide tension (ETCO2) with an increase in arterial carbon dioxide tension (PaCO2). Both of these features are characteristic of VAE.
c. Hypoxemia develops owing to the partially occluded pulmonary vasculature and the local release of vasoactive substances.
D. Monitoring for VAE. Multiple modalities are available for VAE monitoring including hemodynamic monitors (BP, central venous pressure [CVP], and PA pressure), precordial Doppler ultrasound, end-tidal gas monitoring, and transesophageal echocardiography (TEE). For high-risk cases, Doppler and ETCO2 monitoring are considered the acceptable minimum (Table 9.1).
TABLE 9.1 Comparison of VAE Detection Methods
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






