Genetic syndrome
% Pheo
Features
VHL type 2 a–c
10–20
CNS hemangioblastoma
von Hippel–Lindau syndrome
Renal carcinoma
Chromosome 3
Endolymphatic sac tumors
Epididymal cystadenomas
Pancreatic and renal cysts
Retinal angiomas
Affects also children and adolescents
Pheochromocytoma
Often bilateral and extra-adrenal location
Usually benign; mainly noradrenergic but some adrenergic
MEN 2 A
~50
Medullary thyroid carcinoma
Multiple endocrine neoplasia
Parathyroid adenoma or hyperplasia
RET gene
Hyperparathyroidism
Chromosome 10
Hirschsprung’s disease
Cutaneous lichen amyloidosis
Affects also children and adolescents
Pheochromocytoma; often bilateral but rarely extra-adrenal. Usually benign. Often adrenergic
MEN 2 B
~50
Medullary thyroid carcinoma
Multiple endocrine neoplasia
Marfanoid habitus
RET gene
Intestinal ganglioneuromas
Chromosome 10
Neuromas of tongue and lips
Conjunctiva nerve hyperplasia
Affects also children and adolescents
Pheochromocytoma; often bilateral but rarely extra-adrenal. Usually benign. Often adrenergic
PGL 4 SDHB chromosome 1
20
Head and neck paraganglioma
Familial paraganglioma
Renal carcinoma
Sympathetic functional tumors
Noradrenergic or dopaminergic
Multifocal/increased extra-adrenal location
Increased risk of malignancy 35–70%
PGL 3 SDHC chromosome 1
?
Head and neck paraganglioma (chemodectoma)
PGL 2 SDH5 chromosome 11
Parasympathetic nonfunctional tumors
PGL 1 SDHD chromosome 11
Multifocal/increased extra-adrenal location
Familial paraganglioma
Occasionally developing catecholamine excess but small chance of malignant disease
NF1 Neurofibromatosis type 1
1
Cafe’-au-lait spots and axillary freckling
Von Recklinghausen
Multiple dermal neurofibromas
Chromosome 17
Lisch nodules of the iris
Optic and other CNS gliomas
Pheochromocytomas are rare, in particular, in children, but in relation frequently malignant
Whereas pheochromocytomas and abdominal paragangliomas are catecholamine-producing tumors of the sympathetic nervous system (SNS), nonfunctional paragangliomas (“head and neck”; chemodectomas, glomus and carotid-body tumors) are nonsecreting tumors of parasympathetic origin [28].
In pheochromocytoma patients, the activity of the sympathetic nervous system may be enhanced due to increased loading of sympathetic vesicles with norepinephrine. The resulting excessive release of postganglionic neuronal norepinephrine during nerve stimulation can result in marked symptoms despite relatively small increments in circulating catecholamines [16, 41]. As a consequence, any eliciting situation [3] that leads to a stimulation of the SNS (e.g., anxiety, pain, invasive procedures) can result in excessive release of transmitter and an exaggerated physiologic response, which can be just as problematic as the unpredictable release of vasoactive hormones from the tumor itself.
Virtually all epinephrine-secreting tumors are adrenal in origin. This is because the converting enzyme, phenylethanolamine-N-methyltransferase (Fig. 8.1), is glucocorticoid dependent and thus found in the adrenal gland. Dopamine-secreting pheochromocytomas are very rare and should, if present, always raise suspicion of malignancy [42]. The clinical features of the rare dopamine-secreting tumors are also of nonspecific “inflammatory” or “hypermetabolic” nature, and the patients are not hypertensive [42]. Secretion of norepinephrine is normally causing hypertension, but when symptoms are those of hypermetabolism, epinephrine co-secretion should be suspected [4]. In pheochromocytoma patients, fasting blood glucose concentration is increased, and the tolerance curve is abnormal. High catecholamine concentrations lead to glycogenolysis, lipolysis, and inhibition of insulin release (α2-agonism). In cases with epinephrine involved, this effect is partly opposed by the β2-agonistic promotion of insulin release [6, 41].
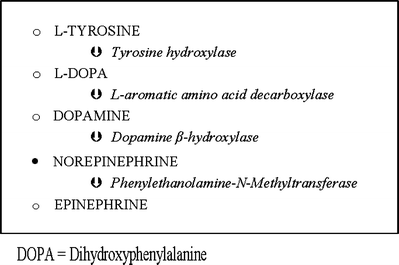

Fig. 8.1
Catecholamine synthesis
Clinical Presentation
The classical triad of pheochromocytoma presentation is paroxysmal sweating, hypertension, and headache. Hypertension is sustained in 50%, paroxysmal in 30%, and blood pressure is normal in 20% of patients. In rare cases when mainly epinephrine or dopamine is secreted, orthostatic hypertension may be the presenting symptom [43]. Further symptoms include weight loss, hyperglycemia, tachycardia or tachyarrhythmia, tremor, pallor, and flushing depending on which catecholamine is secreted. Other clues to the diagnosis are hypertension that is episodic (spells) or difficult to treat, glucose intolerance, nausea, palpitations, and problems with blood pressure in connection with induction of anesthesia, labor, abdominal examination, surgery, or other forms of stress. A pressor response to particular drugs can also suggest the presence of this tumor. These drugs include histamine, glucagon, droperidol, metoclopramide, tyramine (in food or wine), cytotoxic drugs, saralasin, tricyclic antidepressants and phenothiazines, cocaine, alcohol, ephedrine, ketamine, pancuronium, halothane, morphine, atracurium, and suxamethonium [1]. Intravenous contrast previously was considered a trigger, but recently published experience suggests otherwise [44]. Glucocorticoids can increase catecholamine synthesis in pheochromocytoma cells by inducing biosynthetic enzymes such as phenylethanolamine N-methyltransferase, tyrosine hydroxylase, and dopamine b-hydroxylase [10], which explains the time lag of several hours between the glucocorticoid administration and the onset of hypertension [44].
A sudden increase in sympathetic activity with both neuronal norepinephrine unloading and tumor catecholamine release can cause severe vasoconstriction which may lead to life-threatening pulmonary edema and dysrhythmias [41]. Mortality in pheochromocytoma is usually caused by a malignant hypertensive crisis with cerebrovascular accidents or dissecting aortic aneurysm, myocardial infarction, arrhythmias, heart failure, acute renal failure, or irreversible shock leading to multiple organ dysfunction. Pheochromocytoma cells may also release other peptides, some of which cause symptoms that cannot be controlled by adrenergic blockade only. Such secretory products include substance P, neuropeptide Y, enkephalins, somatostatin-corticotropin-releasing hormone, adrenocorticotropin hormone, atrial natriuretic peptide, vasointestinal peptide, parathormone, interleukin-1, interleukin-6, calcitonin gene-related peptide, and chromogranin A [41].
Diagnosis
Provocation or suppression tests are not often used in modern practice. In the common clinical setting, measurement of 24-h urinary metanephrines may be best screening due to low likelihood of false positive results. In patients at high risk of having pheochromocytoma, measurements of fractionated plasma metanephrines may be preferable as its sensitivity approaches 100% [26, 29]. The introduction of HPLC (high-pressure liquid chromatography) methods has largely removed the problem of drug and dietary interference affecting the results [15].
It is important to first appreciate that under normal conditions, catecholamines released by nerve cells are mainly subject to neuronal reuptake [24]. Only minor amounts are metabolized or escape into circulation. The first step of metabolism (Fig. 8.2) is deamination, but in the adrenal medulla where catechol-O-methyltransferase (COMT) is present, methylation results in the formation of metanephrines. Other intermediate metabolites undergo conjugation to glucuronides and sulfates that are excreted in the urine.
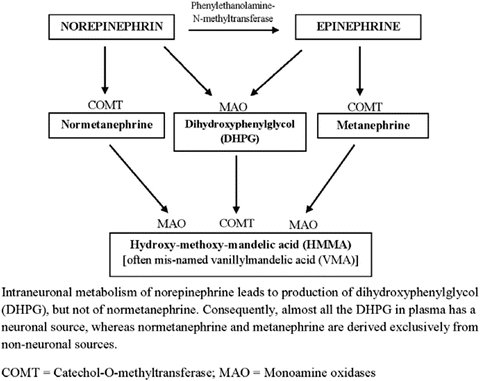

Fig. 8.2
Major catecholamine metabolism
Dopamine metabolism normally constitutes just a minor pathway (Fig. 8.3). A negative feedback mechanism regulates catecholamine synthesis via tyrosine hydroxylase in normal adrenal medullas but not in pheochromocytomas where the enzyme activity also is much higher.
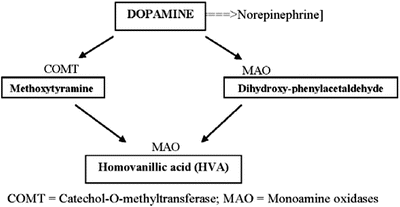

Fig. 8.3
Dopamine metabolism
In contrast to sympathetic nerves that contain monoamine oxidase (MAO), pheochromocytoma cells contain both MAO and high concentrations of membrane-bound COMT. The latter explains the abundance of methylated free metanephrines in plasma from these patients. The continuous intratumoral production of metanephrines makes possible the detection of pheochromocytomas in patients with normal plasma or urinary levels of catecholamines. In a recent review [45] and a report from an international symposium [46], it was suggested that screening with urine 24-h fractionated total metanephrines can be confirmed by analysis of plasma fractionated free metanephrines and plasma chromogranin A or the use of the clonidine suppression test.
Once diagnosis of pheochromocytoma is confirmed by biochemical testing, imaging techniques are employed for tumor localization. Pheochromocytomas are typically large tumors (2–5 cm in diameter) and may contain areas of hemorrhage or necrosis. Tumors in hereditary syndromes tend to be smaller and bilateral. Most tumors are intra-abdominal and 90% originate within the adrenal gland [43]. CT scanning has good sensitivity (93–100%) for detection of adrenal pheochromocytomas, but sensitivity decreases for extra-adrenal tumors. MRI is superior to CT for detecting extra-adrenal tumors and is also used as method of choice in pregnant patients [13]. Functional imaging includes iodinated metaiodobenzylguanidine scanning (131I-MIBG) and 111In-DTPA-octreotide somatostatin-receptor scintigraphy (SRS), but the method of choice currently is 123I-MIBG scintigraphy [46] and the recent development of several positron emission tomography (PET) ligands [50]. Somatostatin receptor imaging might be considered as a supplement for MIBG scintigraphy in pheochromocytoma and paraganglioma patients with suspected metastatic disease [51].
Medical Treatment
Medical treatment is given mainly in line of preparation for surgery. Chemotherapy (e.g., cyclophosphamide, vincristine, and dacarbazine) is currently the treatment of choice for inoperable tumors [50]. Patients that show positive testing for somatostatin receptors and positive scintigraphy may benefit from targeted chemotherapy. Irradiation with radiolabeled MIBG was used for malignant tumors with or without metastases. Even with good initial regression of tumors, no long-lasting effects could be shown so far for either approach [16]. Metyrosine (α-methylparatyrosine) inhibits tyrosine hydroxylase and may decrease catecholamine synthesis by up to 80%. It is very effective but used mainly in malignant or inoperable cases because of the many side effects (sedative fatigue, anxiety, depression, extrapyramidal signs, and tremor).
Surgery
The treatment of choice for adrenal tumor in general is surgical resection once the tumor has reached a certain size (>3–5 cm), becomes symptomatic, or if imaging, genetic testing, or history is suspicious for malignancy [52]. For secreting pheochromocytomas, less invasive techniques like arterial embolization, chemoembolization, cryotherapy, and radiofrequency ablation are normally not considered safe to use, as catecholamine release and its circulatory effects may be difficult to predict and control. Traditionally, open surgery was performed, but since the first report of laparoscopic adrenalectomy for pheochromocytoma in 1992, practices have changed. Several studies have shown that the two techniques are comparable with regards to intraoperative hemodynamic changes, but the postoperative recovery is faster for the laparoscopic approach [53–55]. In many centers, laparoscopic adrenalectomy is the preferred technique for pheochromocytomas and all other benign tumors of up to a size of 6–8 cm [56–59] or bigger [60]. An Italian Registry, with 833 patients undergoing surgery since 2000, concluded that the main risk factors for the occurrence of complications during laparoscopic adrenalectomy appear to be surgical inexperience, the age and BMI of the patient, and the size and nature of the tumor [61]. One recommendation was that laparoscopic removal of these tumors should be undertaken only in high-volume specialty centers by surgical teams with the appropriate training and experience with adrenalectomies [62]. Laparoscopic adrenalectomy has been compared with the posterior retroperitoneoscopic technique in a study on 46 patients. It was concluded that both methods are safe but that the retroperitoneoscopic approach decreased operative times, blood loss, and postoperative length of stay [63]. The two different approaches for retroperitoneoscopic adrenalectomy have also been compared, and both the lateral and posterior techniques have a similar perioperative outcome when patients are selected for each option on predefined criteria [64]. Adrenocortical-sparing surgery may be performed using laparoscopy in patients with hereditary forms of pheochromocytoma [65].
The laparoscopic technique has also been used with success in patients that presented with malignant hypertension and acute heart failure. A release of norepinephrine was elicited by pneumoperitoneum but hypertension could be controlled safely even in this type of patients [66]. However, there are also reports of pneumoperitoneum causing massive norepinephrine release leading to acute heart failure despite treatment with α1-, β-, and calcium channel blockers [67].
Catecholamine-Induced Cardiomyopathy
Sustained norepinephrine release over months or years will lead to hypertrophic cardiomyopathy in 20–30% of patients, the condition being at least partially reversible by the use of adrenergic blockade and tumor removal. Patients may present with symptoms ranging from palpitations and nonspecific electrocardiogram (ECG) – changes to severe dysrhythmias and congestive heart failure. The myocardial dysfunction may be secondary to activation (or down regulation) of adrenoreceptors, coronary vasospasm, or relative ischemia due to hypertrophy and increased myocardial oxygen demands [8]. Even young patients are at risk of developing myocardial ischemia or sustaining a myocardial infarction [68]. Microscopy shows interstitial edema, hemorrhage, and inflammatory infiltrates. The criteria for myocarditis are normally not met. The myocytes show contraction-band necrosis, and later fibrosis and calcification may follow [6]. Intracellular calcium overload appears to be the main abnormality involved [69]. Catecholamines have been shown [70] to influence the extracellular matrix with collagen deposition and subsequent fibrosis in the arterial wall and in the myocardium. These morpho-functional changes can be emphasized by ultrasound imaging. A total of 15 patients were included (hypertension in 10) in a recent study [71]. All but one had a normal left ventricular ejection fraction, however, with a depressed systolic strain rate detected by tissue Doppler echocardiography and an increased risk of intraoperative collapse.
With tailored vasoactive support, congestive heart failure may resolve within a week, and the cardiomyopathy will to some extent reverse over a few months [13, 72–74]. In one case report [48], pretreatment leads to a dramatic reversal of catecholamine-induced cardiomyopathy in less than 2 weeks, i.e., even before resection of the tumor.
Takotsubo cardiomyopathy (“broken-heart syndrome” or “stress-cardiomyopathy”) is an increasingly recognized clinical syndrome of transient left ventricular dysfunction, commonly with apical ballooning. An inverted-Takotsubo contractile pattern is now increasingly associated with pheochromocytoma [75].
Patients presenting with Takotsubo type heart failure should not be administered inotropic agents [76] because of the integral adrenergic mechanism in the pathophysiology of the syndrome. An initial proper treatment could be implantation of an intra-aortic balloon pump counterpulsation to avoid administration of inotropic agents. Further, medications like β-blockers can be used to attenuate the exaggerated stress reaction and carvedilol as α- and β-blocking agent, might be especially useful in patients with Takotsubo syndrome. Also calcium sensitizing medications such as levosimendan could be of use to counter the calcium overload.
Anesthetic and Perioperative Aspects
For any patient with VHL or MEN II A/B presenting for surgery, the diagnosis of pheochromocytoma should be suspected even if the patient is asymptomatic. Additionally, patients who have had pheochromocytoma previously resected, and are returning for surgery, should be screened for recurrences and/or for pheochromocytoma on the unresected side [77]. While extra-adrenal pheochromocytomas are rare in MEN, such tumors should be part of the screening for VHL patients. Examination should aim to uncover any signs of pheochromocytoma sequelae.
The presence of symptomatic cardiac dysfunction will have an impact on both selection and duration of pretreatment and intraoperative management. If catecholamine secretion remains uncontrolled, a life-threatening crisis may develop [78]. The pressor effect will cause end-organ damage such as hypertensive encephalopathy and worsening cardiomyopathy. Since the introduction of aggressive antihypertensive treatment, this crisis is far less commonly seen, but during surgery and other tumor interventions, one should always be prepared to manage acute episodes of hypertension [6]. The events of greatest concern are anesthesia induction, insufflation of pneumoperitoneum, tumor manipulation, and loss of endogenous catecholamine stimulation upon tumor ligation in combination with residual α1-adrenergic blockade after tumor removal. Tumor manipulation is the main risk factor during adrenalectomy since large amounts of catecholamines are released to the circulation with plasma concentration in some patients exceeding normal values by a factor of more than 1,000. Although specific anesthetic drugs have been recommended, the most important factors still are optimal preoperative preparation, gentle induction of anesthesia, and good communication between the surgeon and anesthesiologist. Virtually, all anesthetic drugs and techniques (including isoflurane, sevoflurane, sufentanil, remifentanil, fentanyl, and regional anesthesia) have been used with satisfactory result [1].
Preoperative Optimization
No controlled, randomized, prospective clinical studies have investigated the value of pretreatment with adrenergic receptor blocking drugs. It is often forgotten that the use of phenoxybenzamine in the original publication [18] was for 3 days only prior to surgery. These α-blocking drugs probably reduce the incidence of hypertensive crisis, the wide blood pressure fluctuations during manipulation of the tumor and the myocardial dysfunction that occur perioperatively. The reduction in perioperative mortality (from ~50% to the current 0–3%) is often used as an indirect proof of its efficacy. The α-adrenergic receptor blockade restores plasma volume by counteracting the vasoconstrictive effects of high levels of norepinephrine. For patients who exhibit ST-T changes on electrocardiogram (ECG), long-term (1–6 months) preoperative α-adrenergic receptor blockade has produced ECG normalization and clinical resolution of catecholamine-induced cardiomyopathy [79]. The optimal duration of preoperative therapy with phenoxybenzamine has not been studied. Criteria for the treatment have been recommended [1]. Accordingly, one should aim at a blood pressure of not higher than 165/90 mmHg and with an orthostatic hypotensive response present. The ECG should be free of related ST-T changes that are not permanent and of frequent premature ventricular contractions or symptomatic dysrhythmias.
In many countries, there is still dogmatic insistence on the use of phenoxybenzamine for at least 2 weeks preoperatively, although many experts find a shorter treatment period adequate. The length of treatment can be tailored to the patient’s condition [3]. A few days of treatment to allow reregulation of adrenergic receptors is sufficient for some, whereas prolonged treatment may be necessary to facilitate myocardial remodeling in case of severe hypertrophy of the heart or cardiac dysfunction. Some authors concluded that advances in anesthetic and monitoring techniques and the availability of fast-acting drugs capable of correcting sudden changes in cardiovascular variables have eliminated the need for the use of phenoxybenzamine or other drugs to produce profound and long-lasting α-blockade [20, 21]. In one study [80], patients who were treated with phenoxybenzamine for more than 10 days did not have better perioperative stability than patients who had treatment for less than a week. Nor did the degree of postural hypotension after pretreatment predict operative stability.
A study evaluated the predictive value of preoperative high systolic arterial pressure (SAP) on intra- and postoperative hemodynamic instability in 96 patients undergoing laparoscopic adrenalectomy for pheochromocytoma [81]. It was concluded that for most patients scheduled for laparoscopic pheochromocytoma removal, surgery can be carried out even without systematic preoperative arterial pressure normalization. Some patients, however, must receive hypotensive drugs before surgery to control various hypertension-associated organ dysfunctions such as left ventricular failure or neurological deficit of central origin, or symptoms such as headache or tinnitus. The data from this relatively large series do not support the concept of consistent preoperative SAP normalization. A prospective adequately powered study is mandatory for confirmation of these data [81]. A recent study of 59 patients compared the intraoperative hemodynamics in normotensive pheochromocytoma patients undergoing tumor resection with or without preoperative a-blockade. The authors concluded that pretreatment had no benefit in maintaining intraoperative hemodynamic stability in patients with normotensive pheochromocytoma, and there was an increased use of vasoactive drugs and colloid infusions in the pretreated group [82].
Alternative drugs should be considered for pretreatment of patients with congestive heart failure in whom α-adrenergic blockade leads to tachycardia and β-adrenergic blockade diminishes cardiac performance [13]. In a report of two complicated cases, it is pointed out how important it is that the anesthesiologist carefully monitors the endpoints of the patient’s pretreatment and alerts the team of potential cardiovascular risk factors that may impact the intraoperative course [83]. In these reports, the patients had large pheochromocytomas, preoperatively insufficiently controlled blood pressure, and/or myocardial dysfunction. The use of β-adrenergic blockade prior to surgery might also have increased the patient’s vulnerability to untoward events later on, including intractable hypotension, bradycardia, and asystolic cardiac arrest. Preoperatively it was noted that cardiac compromise secondary to volume overload occurred, and intraoperatively massive doses of exogenous catecholamines were needed post-ligation.
Author’s opinion on preoperative optimization is expressed in Table 8.2 and is supported in the current literature [84]. This prospective follow-up study of 35 pheochromocytoma patients systematically documents the reversibility of cardiovascular dysfunction. In the study were used serial assessments with ECHO, tissue Doppler imaging (TDI) and serum N-terminal pro-brain natriuretic peptide (NTpro-BNP) to evaluate cardiac function. Seven of the 35 pheochromocytoma patients (20%) were found to have significant LV systolic dysfunction (as defined by LVEF <45%, MPI (Doppler-derived performance index) >0.4, s-NTpro-BNP >500 pg/mL). Normalization ensued within 3 months in most cases.
Table 8.2
Preoperative optimization
Author’s approach to preoperative treatment | |||
|---|---|---|---|
Patient category/risk group | Incidence | Drug = drug added as required | Duration |
No hypertension or pheochromocytoma symptoms/low risk | ~40% | No treatment | – |
Or | |||
α-blocker (doxazosin) | ≤1 week | ||
Hypertension and/or pheochromocytoma symptoms/intermediate risk | ~50% | α-blocker (doxazosin) | 1–4 weeks |
+ | |||
Ca-blocker (nicardipine) | Depending on severity of HT and degree of left ventricular hypertrophy | ||
+ | |||
β-blocker (atenolol) | |||
Symptomatic cardiac disease/high risk | ~10% | α-blocker (doxazosin) | 4–8 weeks |
+ | |||
Ca-blocker (nicardipine) | |||
+ | |||
ACE-inhibitor (ramipril) | |||
Or | |||
Calcium sensitizer (levosimendan) | As required to improve left ventricular compliance | ||
Subtle myocardial damage is common in pheochromocytoma patients, and it can be detected by using biomarkers and/or tissue Doppler imaging despite an absence of overt LV dysfunction. Even if the clinical relevance of the combination of normal ECHO and positive other markers is still unclear, it may be assumed, however, that detailed cardiac evaluation may help tailoring preoperative optimization and thereby reducing perioperative morbidity.
α-Adrenergic Blockade
The α-adrenergic blocker phenoxybenzamine became a standard drug for pretreatment soon after the publication of the first series of patients in 1967 [18]. However, the drug has two characteristics that make it less than ideal. First, it is a nonselective α-blocker, so it prevents not only the postsynaptic α1-mediated vasoconstriction but also the presynaptic α2-mediated inhibition of catecholamine release. If tachycardia ensues the patient will need simultaneous β-adrenergic blockade. In patients with severe cardiomyopathy, however, β-blockade has been shown to precipitate cardiac failure [6]. Secondly, phenoxybenzamine is a noncompetitive inhibitor that binds covalently to the α-receptor. This causes more frequent and more resistant postoperative hypotension of longer duration than other alternative therapies. Because of long plasma half-life, the drug should be withheld for at least 12 h before surgery. Common side effects of nonselective α-blockade include postural hypotension, reflex tachycardia, headache, somnolence, constipation, dry mouth, stuffy nose, and nausea [13].
When comparing two series of patients, doxazosin was found to be as effective as phenoxybenzamine in controlling arterial pressure and heart rate both before and after surgery. Doxazosin, which is a selective and competitive blocker, also had fewer undesirable side effects [85]. No significant differences were found in the operative and postoperative blood pressure control and plasma volume when three groups of patients with phenoxybenzamine, prazosin, or doxazosin pretreatment were compared [86]. In other studies, doxazosin used either alone or in combination with a β-blocker produced excellent hemodynamic control with only minor and transient adverse reactions [39, 87]. In normotensive patients, no blockade of any form was instituted [88]. Another retrospective analysis of risk factors for hemodynamic instability during surgical resection of pheochromocytoma was preformed on 73 patients who underwent surgery between 1995 and 2007. Phenoxybenzamine was used for pretreatment before 2003 and doxazosin from 2003 onwards, and both treatments showed similar efficacy with respect to intraoperative hemodynamic control. Neither was a difference seen in hemodynamic instability nor intraoperative drug administration between the laparoscopic and open or converted procedures [89]. A retrospective chart review was published on 50 Mayo Clinic patients and 37 Cleveland Clinic patients who had undergone laparoscopic pheochromocytoma resection. The respective Clinic predominantly used either phenoxybenzamine or selective α1-blockade. No clinically significant outcome differences were noted, and the use of phenoxybenzamine appeared to produce better attenuation of intraoperative hypertension but at the cost of longer-lasting intraoperative hypotension that required a greater use of vasopressors [90].
Recently, the intravenous use of the selective α1-receptorblocker urapidil (t½ ~ 3 h) for pretreatment was described. The drug replaced prazosin and bisoprolol for 3 days before surgery and was maintained throughout anesthesia. Hypertensive peaks were handled with boluses of nicardipine and esmolol as required. It was concluded that it was safe to use urapidil for perioperative control of blood pressure [91]. In another report, urapidil and magnesium sulfate were used both pre- and intraoperatively in one patient with good result [92].
Currently, there is no consensus for when adrenergic blockade should be started, but in most medical centers, adrenergic blockade usually starts 7–14 days preoperatively to have adequate time to normalize blood pressure and heart rate and to expand the contracted blood volume [27, 54–58]. Preoperative antihypertensive treatment is warranted for patients with organ damage from long-standing catecholamine excess or life-threatening complications of high blood pressure (cardiomyopathy, congestive heart failure, stroke, coronary artery disease, dysrhythmia) and for patients with pheochromocytoma diagnosed during pregnancy [93]. Exceptions where treatment may not be required for blood pressure and heart rate control include patients with parasympathetic-derived head and neck paragangliomas that do not produce catecholamines or patients with very rare tumors producing only dopamine [49].
Calcium Channel Blockade
Several calcium channel blockers have been used in the preoperative preparation of patients with pheochromocytoma. Many years ago, a case was reported where nifedipine was used for pretreatment of a patient with hypertrophic cardiomyopathy [94]. In another case, diltiazem was used preoperatively in a patient with hypertensive crisis due to hepatic metastases from a pheochromocytoma. Intraoperatively, great fluctuations in blood pressure were noted [95]. In a study of 113 patients, calcium channel blockers were used as the primary mode of antihypertensive therapy with good result. Selective α-antagonists were added only if the hypertension was not adequately controlled, and β-blocker was used where cardiac dysrhythmias were noted. One of the most effective calcium channel blocker appears to be nicardipine. In several series of patients, pretreatment with nicardipine was successful with little need for additional drugs to control hypertension and without the risk of prolonged hypotension after tumor removal [54, 96, 97]. However, the putative mechanism – prevention of increased free plasma catecholamine levels – could not be demonstrated [97]. Calcium channel blockers also proved safe in laparoscopic adrenalectomy when compared with groups treated with α-blockers and/or β-blockers [53]. In a retrospectively studied series of more than 100 patients, the use of nicardipine (pre- and perioperative) was associated with low mortality and morbidity even when not all hemodynamic changes were prevented [98].
β-Adrenergic Blockade
The use of β-adrenergic receptor blockade has been suggested for patients who have persistent dysrhythmias or tachycardia (often epinephrine or dopamine secretion), because these conditions can be precipitated or aggravated by nonselective α-adrenergic receptor blockade. Similarly, nonselective β-blockade, when given before α-blockade in case of norepinephrine-secreting tumor, can give rise to an unopposed vasoconstrictor effect. This can increase the risk of dangerous hypertension. The same phenomenon can occur when labetalol is used [99]. The short acting β-blocker esmolol was successfully used in combination with sodium nitroprusside to control circulation during surgery for pheochromocytoma [100]. Onset of esmolol is rapid and its effect largely reversed within 30 min. Recently the use of landiolol, an even shorter acting and more highly β1-selective adrenergic blocker, was reported for treating intraoperative tachyarrhythmia [101].
Metyrosine (See Medical Treatment)
Tumors secreting epinephrine, and in particular dopamine, are very rare. In non-hypertensive patients with this kind of tumors, no preoperative α-antagonists are given [102] as they can worsen unopposed β-adrenergic activity. If pretreatment is necessary for arrhythmias or other symptoms that do not respond to β-blockers, metyrosine can be tried as it blocks the conversion of tyrosine to the dopamine precursor DOPA.
Magnesium
Although magnesium sulfate has been used for preoperative preparation, its main application is intraoperatively. This drug is described further in the section on intraoperative management.
Intraoperative Management
Premedication
All usual preoperative medication should be continued but if phenoxybenzamine is used it is normally stopped the day before surgery. Preventing stress is very important, and a benzodiazepine is a good choice for anxiolysis.
Monitoring
Intra-arterial pressure recording should be started and a large-bore venous catheter inserted prior to induction of anesthesia and continued into the postoperative period. Monitoring of 5-lead ECG, ventilation, arterial blood gases, blood glucose concentration, urine output, and body temperature are all also part of routine.
Induction
Most routinely available techniques can be used, but drugs known to release histamine are best avoided. The selective α2-adrenoceptor agonist dexmedetomidine can be used for its sedative and analgesic properties to attenuate sympathoadrenal responses to tracheal intubation and intraoperative stimuli [103, 104]. Halothane (Ca-channel inhibition less of a problem with modern inhalational agents) sensitizes the myocardium to the effects of catecholamines and may thus have pro-arrhythmogenic properties. A combination of propofol and a short-acting opioid is considered safe, and lidocaine is sometimes added. For muscle relaxation, vecuronium has advantages in that it is relatively devoid of vagolytic or sympathomimetic effects. Depolarizing relaxants increase intra-abdominal pressure, which might set free catecholamines from the tumor. For rapid sequence induction, one should therefore consider using rocuronium. Ketamine is often avoided because of its mild sympathomimetic effects. Glycopyrrolate would be the first choice if anticholinergic agent is needed and possible atropine-induced tachycardia a concern.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







