CHAPTER 20 Anesthesia for Congenital Heart Surgery
This chapter describes the perioperative management of major forms of congenital heart disease (CHD) that require surgery with the use of cardiopulmonary bypass. Congenital cardiac lesions that are primarily addressed in the catheterization laboratory or without CPB are discussed in Chapter 21, Congenital Cardiac Anesthesia: Non-Bypass Procedures.
Congenital anomalies of the heart and cardiovascular system occur in 7 to 10 per 1000 live births. Congenital heart disease is the most common congenital disease, accounting for approximately 30% of all congenital diseases. CHD has become the principal cause of pediatric heart disease as the incidence of rheumatic heart disease has declined. Ten percent to 15% of children with CHD have associated congenital anomalies of the skeletal, genitourinary, or gastrointestinal system. The U.S. population of adults with CHD, surgically corrected or uncorrected, is estimated to exceed 1 million and is increasing steadily. As a result, it is not uncommon for adult patients with CHD to present for noncardiac surgery (see Chapter 21, Congenital Cardiac Anesthesia: Non-Bypass Procedures).
Congenital heart disease can be associated with specific complications. For example, infective endocarditis is a risk associated with most congenital cardiac anomalies. Sudden death occasionally occurs in patients who undergo surgical correction of CHD, presumably reflecting the effects of chronic abnormal hemodynamic loads, myocardial scarring and fibrosis, damage to the cardiac conduction system, or underlying (and presently occult) abnormal molecular and ion channel defects. Cardiac dysrhythmias are not usually a prominent presenting feature of CHD but can be more common as patients age and pathophysiologic sequelae of abnormal cardiac structure, function, and surgery accrue (see later and Chapter 21, Congenital Cardiac Anesthesia: Non-Bypass Procedures). Table 20-1 summarizes the pathophysiology and clinical picture associated with a wide variety of congenital heart defects. Table 20-2 summarizes the surgical repair options for each type of lesion.
TABLE 20-1 Pathophysiology and Clinical Picture of Congenital Heart Defects
| Lesion Type | Pathophysiology | Clinical Signs and Symptoms |
| Shunt Lesion without Outflow Tract Obstruction | ||
| Shunt Lesions with Right Ventricular Outflow Tract Obstruction | ||
| Transposition Physiology (Intercirculatory mixing) | ||
| Single Ventricle Physiology | ||
| One-Ventricle Lesions | ||
| Two-Ventricle Lesions | ||
| Left Ventricular Obstructive Lesions | ||
| Mitral Stenosis | ||
| Aortic Stenosis | ||
| Coarctation | ||
| Mixing of Systemic and Pulmonary Venous Blood with Series Circulation | ||
| CHF • Systemic and pulmonary vascular congestion; pulmonary vascular congestion is severe if pulmonary venous obstruction PAPVR TAPVR | ||
CHF, Congestive heart failure; PAPVR, partial anomalous pulmonary venous return; TAPVR, total anomalous pulmonary venous return.
TABLE 20-2 Classification of Congenital Heart Lesions and Associated Repairs
| Lesion Type | Repair |
| Shunt Lesions | |
| Left-to-Right | |
| Complete repair | |
| Complete repair | |
| Complete repair | |
| Complete repair | |
| Complete repair | |
| Right-to-Left | |
| Complete repair | |
| Complete repair | |
| Complete repair | |
| No repair | |
| Transposition Physiology | |
| Complete repair | |
| Single-Ventricle Physiology | |
| One-Ventricle Lesions | |
| Staging to Fontan | |
| Staging to Fontan | |
| Staging to Fontan | |
| Two-Ventricle Lesions | |
| Complete repair | |
| Complete repair | |
| Complete repair | |
| Left Ventricular Obstructive Lesions | |
| Mitral Stenosis | |
| Complete repair | |
| Aortic Stenosis | |
| Complete repair | |
| Coarctation | |
| Repair with likely residual lesions | |
| Mixing of Systemic and Pulmonary Venous Blood with Series Circulation | |
| Complete repair | |
Pathophysiology of congenital heart disease
Shunting
Total pulmonary blood flow (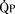 ) is the sum of effective pulmonary blood flow and recirculated pulmonary blood flow. Total systemic blood flow (
) is the sum of effective pulmonary blood flow and recirculated pulmonary blood flow. Total systemic blood flow (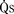 ) is the sum of effective systemic blood flow and recirculated systemic blood flow. Total pulmonary blood flow and total systemic blood flow do not have to be equal. Therefore, it is best to think of recirculated flow (physiologic shunt flow) as the extra, noneffective flow superimposed on the nutritive effective blood flow. These concepts are illustrated in Figures 20-1 to 20-3.
) is the sum of effective systemic blood flow and recirculated systemic blood flow. Total pulmonary blood flow and total systemic blood flow do not have to be equal. Therefore, it is best to think of recirculated flow (physiologic shunt flow) as the extra, noneffective flow superimposed on the nutritive effective blood flow. These concepts are illustrated in Figures 20-1 to 20-3.
Single-Ventricle Physiology
Table 20-3 lists a number of single-ventricle physiology lesions. All patients with single-ventricle physiology who have severe hypoplasia of one ventricle will ultimately undergo the staged surgeries that comprise the single-ventricle pathway and result in Fontan physiology (described later). Patients with single-ventricle physiology and two well-formed ventricles are usually able to undergo a two-ventricle repair. In some cases, the two-ventricle repair will be complete. In others, significant residual lesions (VSD, aortopulmonary collaterals) will remain. In patients with single-ventricle physiology, the arterial oxygen saturation (Sao2) is determined by the relative volumes and saturations of pulmonary venous and systemic venous blood flows that have mixed and reach the aorta (see Fig. 20-1).
TABLE 20-3 Anatomic Subtypes of Single-Ventricle Physiology
| Aortic Blood Flow from: | Pulmonary Artery Blood Flow from: | |
| HLHS | PDA | RV |
| Severe neonatal aortic stenosis | PDA | RV |
| IAA | LV (proximal)PDA (distal) | RV |
| PA with IVS | LV | PDA |
| Tetralogy of Fallot with pulmonary atresia | LV | PDA, MAPCAs |
| Tricuspid atresia, NRGA, with pulmonary atresia (type 1A) | LV | PDA, MAPCAs |
| Tricuspid atresia, NRGA, with restrictive VSD and pulmonary stenosis (type 1B) | LV | LV thru VSD to RV |
| Tricuspid atresia, NRGA, with non-restrictive VSD and no pulmonary stenosis (type 1C) | LV | LV thru VSD to RV |
| Truncus arteriosus | LV and RV | Aorta |
| DILV, NRGA | LV | LV thru BVF |
BVF, Bulboventricular foramen; DILV, double-inlet left ventricle; HLHS, hypoplastic left heart syndrome; IAA, interrupted aortic arch; LV, left ventricle; MAPCAs, multiple aortopulmonary collateral arteries; NRGA, normally related great arteries; PA with IVS, pulmonary atresia with intact ventricular septum; PDA, patent ductus arteriosus; RV, right ventricle; VSD, ventricular septal defect.
Intercirculatory Mixing
Intercirculatory mixing is the unique situation that exists in transposition of the great arteries (TGA) (see Fig. 20-2). In TGA, there are two parallel circulations because of the existence of atrioventricular concordance (right atrium to right ventricle [RA-RV], and left atrium to left ventricle [LA-LV]) and ventriculoarterial discordance (right atrium to aorta [RV-Ao], and left ventricle to pulmonary artery [LV-PA]). This produces a parallel rather than a normal series circulation. In this arrangement, parallel recirculation of pulmonary venous blood in the pulmonary circuit and systemic venous blood in the systemic circuit occurs. Therefore, the physiologic shunt or the percentage of venous blood from one system that recirculates in the arterial outflow of the same system is 100% for both circuits.
Thus, this lesion is incompatible with life unless there are one or more communications (atrial septal defect [ASD], patent foramen ovale [PFO], VSD, PDA) between the parallel circuits to allow intercirculatory mixing. In the presence of mixing, arterial saturation (Sao2) is determined by the relative volumes and saturations of the recirculated systemic and effective systemic venous blood flows reaching the aorta (see Fig. 20-3).
Fontan Physiology
Fontan physiology (see also later) is a series (i.e., “normal”) circulation in which one ventricle has sufficient diastolic, systolic, and atrioventricular valve function to support systemic circulation (Figs. 20-4 and 20-5). This ventricle must in turn be in unobstructed continuity with the aorta and pulmonary venous blood return; there must also be unobstructed delivery of systemic venous blood to the pulmonary circulation (total cavopulmonary continuity).
General Approach to Anesthetic Management
Preparation for Anesthesia
Sevoflurane, halothane, isoflurane, and fentanyl plus midazolam do not change the ratio of pulmonary-to-systemic blood flow (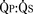 ) in children with atrial and ventricular septal defects when cautiously administered with 100% oxygen (Laird et al., 2002). Sevoflurane (1 minimum alveolar concentration [MAC]) and fentanyl plus midazolam have no significant effect on myocardial function in patients with a single ventricle (Ikemba et al., 2004). Halothane (1 and 1.5 MAC) depresses cardiac index and contractility more than comparable levels of sevoflurane, isoflurane, and fentanyl plus midazolam anesthesia (Rivenes et al., 2001). In addition, halothane anesthesia may result in more severe hypotension and emergent drug use than sevoflurane anesthesia in children with CHD (Russell et al., 2001).
) in children with atrial and ventricular septal defects when cautiously administered with 100% oxygen (Laird et al., 2002). Sevoflurane (1 minimum alveolar concentration [MAC]) and fentanyl plus midazolam have no significant effect on myocardial function in patients with a single ventricle (Ikemba et al., 2004). Halothane (1 and 1.5 MAC) depresses cardiac index and contractility more than comparable levels of sevoflurane, isoflurane, and fentanyl plus midazolam anesthesia (Rivenes et al., 2001). In addition, halothane anesthesia may result in more severe hypotension and emergent drug use than sevoflurane anesthesia in children with CHD (Russell et al., 2001).
Intravenous Induction
Many of these patients come to the operating room with functioning intravenous (IV) access. For those without it, effective premedication may facilitate IV placement and allow the attendant risks of mask induction in this population to be avoided. In some patients, oral midazolam (0.5 to 1.0 mg/kg) may suffice. Others have used oral combinations of meperidine (3 mg/kg) and pentobarbital (4 mg/kg) successfully in this group of patients (Nicolson et al., 1989). Ketamine (∼3 to 6 mg/kg) and midazolam (1 mg/kg) given orally in combination can be quite effective in terms of producing deep sedation and conditions favorable for IV placement and subsequent intravenous induction (Auden et al., 2000).
High-dosage synthetic narcotics in combination with pancuronium (0.1 mg/kg) are commonly used for intravenous induction in neonates and infants. The vagolytic and sympathomimetic effects of pancuronium counteract the vagotonic effect of synthetic opioids. In patients with a low aortic diastolic blood pressure and a high baseline heart rate, vecuronium (0.1 mg/kg) or cisatracurium (0.2 mg/kg) may be used without affecting heart rate. In older children with mild to moderately depressed systolic function, lower dosages of a synthetic opioid can be used in conjunction with etomidate (0.1 to 0.3 mg/kg) (Sarkar et al., 2005).
Ketamine (1 to 2 mg/kg) is a useful induction agent. For patients with both normal and elevated baseline pulmonary vascular resistance (PVR), ketamine causes minimal increases in pulmonary artery pressure as long as the airway and ventilation are supported (Morray et al., 1984). The tachycardia and increase in systemic vascular resistance (SVR) induced by ketamine may make it unfavorable for use in patients with systemic outflow tract obstructive lesions (Williams et al., 2007).
The myocardial depressive and vasodilatory effects of propofol and thiopental make them largely unsuitable as induction agents except in patients with simple shunt lesions in whom cardiovascular function is preserved (Williams et al., 1999b).
Specific lesions
Atrial Septal Defect
Atrial septal defect accounts for about one third of the congenital heart disease detected in adults, with the frequency in women two to three times that in men. See related video online at www.expertconsult.com.
![]() Strictly speaking, an ASD is a communication between the left and right atrium resulting from a defect in the intraatrial septum, which consists of a central membranous portion and a thicker inferior and superior fatty limbus. The central membranous portion is formed by tissue of the septum primum, ultimately forming the fossa ovalis. This membrane lies posterior to the superior aspect of the fatty limbus.
Strictly speaking, an ASD is a communication between the left and right atrium resulting from a defect in the intraatrial septum, which consists of a central membranous portion and a thicker inferior and superior fatty limbus. The central membranous portion is formed by tissue of the septum primum, ultimately forming the fossa ovalis. This membrane lies posterior to the superior aspect of the fatty limbus.
ASDs are classified by their location (Fig. 20-6). There are four morphologic types: ostium secundum defects, ostium primum defects, inferior and superior sinus venosus defects, and coronary sinus (CS) defects. Although they are classified as ASDs, sinus venosus and CS defects are not truly defects in the intraatrial septum. Secundum ASDs account for 80% of all ASDs. Whereas a PFO results from incomplete fusion of an intact fossa ovalis membrane with the superior aspect of the fatty limbus, an ostium secundum ASD is the result of actual deficiencies in the membrane (septum primum) of the fossa ovalis. Isolated ostium primum ASDs, also known as partial atrioventricular canal defects, are discussed later (see Atrioventricular Canal Defects). The isolated ostium primum defect extends from the inferior intraatrial septum fatty limbus, to the crest of the intact ventricular septum.
Clinical Presentation
Some small ostium secundum ASDs can be primarily closed, whereas larger defects are patched, usually with pericardium. An alternative is nonoperative device closure of secundum ASDs in the cardiac catheterization laboratory (see Chapter 21, Congenital Cardiac Anesthesia: Non-Bypass Procedures). Primum ASDs generally require patch closure and suture closure of the anterior leaflet mitral cleft. Sinus venous defects without partially anomalous pulmonary venous return can be closed primarily with a patch. An alternative procedure is performed when the pulmonary vein or veins anomalously enter the SVC. The SVC is transected above the origin of the anomalous vein or veins, and the SVC orifice is directed across the defect into the LA with a pericardial patch. The distal end of the SVC is then anastomosed in an end-to-end fashion to the roof of the RA appendage, recreating SVC-to-RA continuity.
Management of Anesthesia
The goals of anesthetic management for patients with ASD before cardiopulmonary bypass (CPB) follow from the aforementioned principles and are outlined in Box 20-1. The goals for these patients after CPB are outlined in Box 20-2.
Box 20-1 Anesthesia Management Goals for ASD, VSD, AVC Defect, and PDA before Cardiopulmonary Bypass (CPB)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



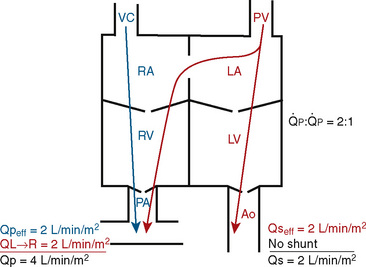
 FIGURE 20-1
FIGURE 20-1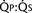 (ratio of total pulmonary to total systemic blood flow) is 2:1.
(ratio of total pulmonary to total systemic blood flow) is 2:1. 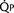 (4.0 L/min per meter-squared) is the sum of the effective pulmonary blood flow (2.0 L/min/m2) and a L-R physiologic shunt (2.0 L/min/m2). This L-R shunt is recirculation of pulmonary venous blood into the pulmonary artery that imposes a volume load on the left atrium, right atrium, and right ventricle.
(4.0 L/min per meter-squared) is the sum of the effective pulmonary blood flow (2.0 L/min/m2) and a L-R physiologic shunt (2.0 L/min/m2). This L-R shunt is recirculation of pulmonary venous blood into the pulmonary artery that imposes a volume load on the left atrium, right atrium, and right ventricle.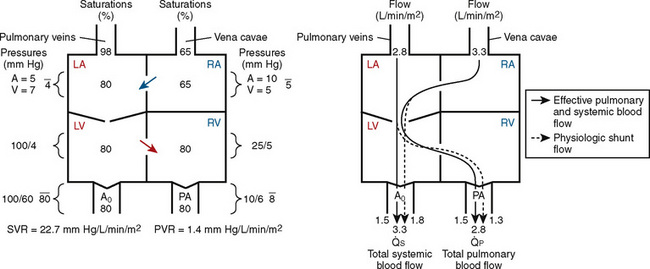
 FIGURE 20-2
FIGURE 20-2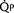 ) is 2.8 L/min/m2 and is the sum of effective pulmonary blood flow (1.5 L/min/m2) and a physiologic and anatomic L-R shunt (1.3 L/min/m2) at the VSD. Total systemic blood flow (
) is 2.8 L/min/m2 and is the sum of effective pulmonary blood flow (1.5 L/min/m2) and a physiologic and anatomic L-R shunt (1.3 L/min/m2) at the VSD. Total systemic blood flow (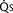 ) is 3.3 L/min/m2 and is the sum of effective systemic blood flow (1.5 L/min/m2) and a physiologic and anatomic R-L shunt (1.8 L/min/m2) at the ASD. Here, there is a small pressure gradient at the atrial level and a large pressure gradient at the ventricular level. In addition, there is a small additional gradient at the level of the pulmonic valve.
) is 3.3 L/min/m2 and is the sum of effective systemic blood flow (1.5 L/min/m2) and a physiologic and anatomic R-L shunt (1.8 L/min/m2) at the ASD. Here, there is a small pressure gradient at the atrial level and a large pressure gradient at the ventricular level. In addition, there is a small additional gradient at the level of the pulmonic valve.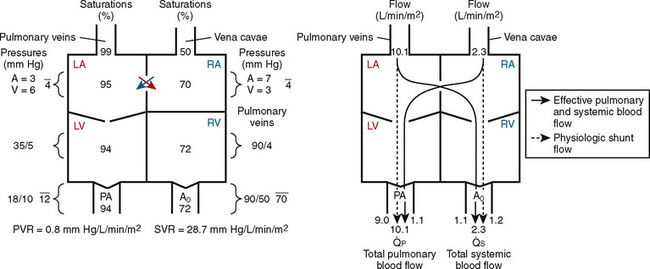
 FIGURE 20-3
FIGURE 20-3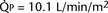 ) is almost five times the total systemic blood flow (
) is almost five times the total systemic blood flow (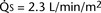 ). The bulk of pulmonary blood flow is recirculated pulmonary venous blood. Here, pulmonary vascular resistance is low (approximately 1/35 of systemic vascular resistance) and there is a small (17 mm Hg, peak to peak) gradient from the left ventricle to the pulmonary artery. These findings are compatible with the high pulmonary blood flow shown.
). The bulk of pulmonary blood flow is recirculated pulmonary venous blood. Here, pulmonary vascular resistance is low (approximately 1/35 of systemic vascular resistance) and there is a small (17 mm Hg, peak to peak) gradient from the left ventricle to the pulmonary artery. These findings are compatible with the high pulmonary blood flow shown.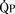 ) and systemic blood flow (
) and systemic blood flow (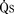 ), (2) distribution of systemic and pulmonary blood flow is dependent on the relative resistances to flow (both intracardiac and extracardiac) into the two parallel circuits, and (3) oxygen saturations are the same in the aorta and the pulmonary artery. This physiology can exist in patients with one well-developed ventricle and one hypoplastic ventricle, as well as in patients with two well-formed ventricles.
), (2) distribution of systemic and pulmonary blood flow is dependent on the relative resistances to flow (both intracardiac and extracardiac) into the two parallel circuits, and (3) oxygen saturations are the same in the aorta and the pulmonary artery. This physiology can exist in patients with one well-developed ventricle and one hypoplastic ventricle, as well as in patients with two well-formed ventricles.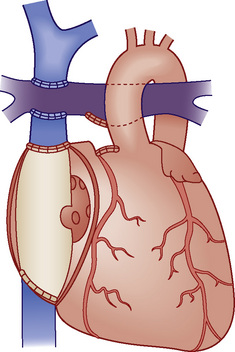
 FIGURE 20-4
FIGURE 20-4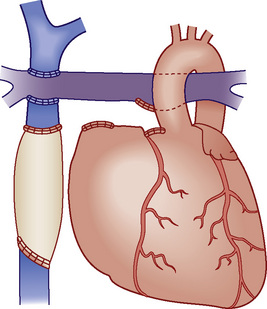
 FIGURE 20-5
FIGURE 20-5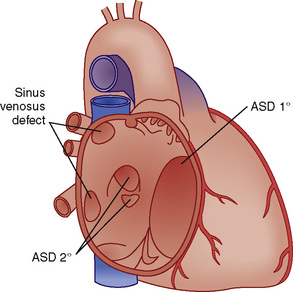
 FIGURE 20-6
FIGURE 20-6




