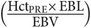Levels of 2,3-DPG versus hemoglobin (Hgb) over time.
Etiology of postoperative anemia
The incidence of preoperative anemia ranges from 11% to 76% depending upon the patient population studied,[2] and anemia will be even more prevalent after surgical blood wastage. Postoperative anemia is an anticipated outcome of surgery. Clinical concern ought to be elicited when the severity of anemia is out of proportion to the reported surgical blood loss. The differential diagnosis of postoperative anemia out of proportion to expectations is narrow, but determining the etiology is crucial to minimizing potential complications.
Inaccurate estimated blood loss (EBL): Surgical blood loss is typically estimated by visual inspection of visible blood loss, gravimetric analysis of fluid content of surgical gauze and sponges, and the measured volume of suctioned fluids. Each of these methods introduces some error into the calculation of EBL. Numerous studies have found substantial inaccuracy in the visual estimation of blood loss and poor inter-individual reliability. This is true whenever estimation of puddled blood is necessary, including child birth,[3–13] skin grafting,[14] trauma,[15,16] and epistaxis therapy.[17] Sponge weight is a more objective measurement, but is also subject to inaccuracy in measurement. The presence of other fluids, including irrigation and urine, will contribute to wet weight, overestimating blood loss, while evaporative fluid loss leads to underestimation of blood loss and is affected by surgical duration, room temperature, and humidity. Other fluids besides blood may be suctioned from the surgical field and the contribution of irrigation fluids or urine to suctioned fluids must be accurately deducted.
EBL may overestimate blood loss at very low volumes, but underestimates blood loss when volumes are high.[18] EBL has been found to vary from actual blood loss by 26% after major orthopedic procedures[19] and as much as 49% after delivery.[20] A significant discrepancy of the anticipated hemoglobin or hematocrit from measured values could be due to an inaccurate EBL. Anticipated hemoglobin in the euvolemic patient after appropriate volume resuscitation may be calculated by the formula in Table 40.1.
| Average blood volume | |
|---|---|
| Adult women | 60–65 ml/kg |
| Adult men | 70–75 ml/kg |
| Infant | 80 ml/kg |
| Full-term neonates | 85–90 ml/kg |
| Premature infants | 95–120 ml/kg |
Dilutional anemia: Measured hemoglobin and hematocrit will reflect red blood cell loss, but also the degree of intravascular volume re-expansion. In the extreme form, rapid blood loss without volume replacement reduces circulatory volume but does not immediately alter hemoglobin or hematocrit. Too little volume replacement with crystalloids (under-resuscitation) results in an artifactually high hemoglobin/hematocrit measurement. Too much volume replacement with crystalloids (over-resuscitation) may result in an artifactually low measurement. Some degree of dilutional anemia is common in the early postoperative setting. Other indicators of hypervolemia may or may not be present.
Occult hemorrhage: Ongoing hemorrhage may be recognized in the postoperative setting by sanguineous seepage on to surgical dressings and output into surgical drains or chest tube. Unanticipated severe postoperative anemia should cause clinical suspicion for occult sources of bleeding. Occult hematoma formation can occur anywhere, including the limbs, thoracic, and retroperitoneal space where a significant proportion of the circulatory blood volume may be lost without clinical notice. Occult hemorrhage may be related to the surgical procedure or trauma, or may be related to other procedures or coincidental to the surgery. These later causes are often easily overlooked. Central line placement or regional anesthetic should raise concern for occult bleeding remote from the surgical site. If a hemothorax has occurred, a chest X-ray may show a large one-sided effusion. Retroperitoneal hematomas can be more challenging to diagnose because the most common signs of retroperitoneal hematomas are non-specific (hypotension and tachycardia). More specific findings in retroperitoneal hematomas are bruising and pain that occur in the peri-umbilical region (Cullen’s sign) or in the flank (Grey Turner’s sign). Abdominal, flank, groin, or lumbar radicular pain may be present. Fatal occult hemorrhage has been reported from lumbar sympathetic block with a 26-gauge needle,[21] so seemingly innocuous clinical interventions can have profound effects if recognition of occult hemorrhage is delayed.
Hemolysis: Although rare, hemolysis is an etiology of disproportionate postoperative anemia. Hemolysis is the premature destruction of erythrocytes. Hemolysis is most frequently a consequence of transfusion reaction, but may occur in surgical patients in the absence of blood transfusion. If hemolysis is suspected, the diagnosis can be confirmed by an abnormal blood smear demonstrating fragment red blood cells (schistocytes), elevated or normal reticulocyte count, elevated bilirubin, elevated lactate dehydrogenase, and a low haptoglobin.
Drug-induced hemolytic anemia can occur by autoimmune or non-autoimmune mechanisms. Over 125 medications have been identified in causing autoimmune hemolytic anemia.[22] The most commonly implicated medications that are frequently administered in the perioperative period are procainamide and antibiotics (especially penicillins and cephalosporins), but perioperative polypharmacy creates some small degree of risk in all surgical patients for this rare clinical phenomenon. Drug-induced non-immune hemolytic anemia is more common and can occur in predisposed individuals with enzymatic deficiencies. The most common is glucose-6-phosphate dehydrogenase (G6PD) deficiency. The highest prevalence of G6PD deficiency is among persons of African, Asian, or Mediterranean descent. Hemolysis is triggered by oxidative stress, including surgery itself and some medications. Methylene blue, sulfa drugs, and nitrofurantoin are unsafe for all patients with G6PD deficiency, but a larger number of medications may also cause hemolysis in individuals with severely deficient enzymatic activity (including diphenhydramine, prilocaine, acetaminophen, aspirin, procainamide).[23] Hemolysis can occur within hours of the exposure but usually is most apparent 24 to 72 hours after the oxidative stress.
Immune and non-immune etiologies of hemolysis can be differentiated with a direct Coombs’ test or direct antiglobulin test (DAT). The DAT is positive in immune-mediated hemolysis. In non-immune-mediated hemolysis, the DAT is negative. The assay test that is performed to confirm a diagnosis of G6PD can be falsely negative during an active hemolytic episode. If G6PD deficiency is suspected, the offending agent should be discontinued, supplemental oxygen should be provided, and the patient referred to outpatient hematology and tested no sooner than 2 to 3 weeks after the hemolytic episode.
Other surgical patients may be at increased risk for hemolysis owing to underlying red blood cell defects. The most clinically common are sickle cell disease and hereditary spherocytosis. Sickle cell disease is an inherited mutation in the β-globin gene causing erythrocytes to form an abnormal sickle or crescent shape. The incidence is about 1 in every 500 African Americans. Red blood cell sickling can occur at any time, but is exacerbated when oxygen concentration in tissues is low. Maintenance of adequate oxygenation, hydration, normothermia, and analgesia are the fundamentals in preventing pain and hemolysis in a sickle cell patient. Hereditary spherocytosis is an inherited disease where the membrane of the erythrocyte is altered to be spherical instead of the normal discoid shape. The disease is encountered worldwide, but is most prevalent in people of northern European descent where it is found in about 0.02% to 0.03% of the population.[24] Affected patients may be completely asymptomatic, severely anemic, or somewhere between these extremes. Anemia is exacerbated during times of stress and viral infections. Findings to support the diagnosis of hereditary spherocytosis include: spherocytes on blood smear, small mean corpuscular volume (MCV), raised mean corpuscular hemoglobin concentration (MCHC), increased reticulocyte count, increased bilirubin, and splenomegaly.
Hemolysis may also be caused by direct red blood cell trauma in unique clinical circumstances. Mechanical valves, particularly ball-valves in the mitral position, and ventricular assist devices may cause hemolysis by this mechanism.
Anemia of chronic disease (AOCD): AOCD may be seen with certain chronic infections, malignancies, and autoimmune disorders. The disruptive effect of interleukin-6 (IL-6) upon iron metabolism has been identified as contributing to the development of anemia. Laboratory testing reveals below-normal hemoglobin, hematocrit, serum iron, serum transferrin, and transferrin saturation, but normal MCV.
A similar pattern of laboratory results consistent with AOCD is seen in patients with postoperative anemia, but the pattern is not observed clinically after hemorrhage unrelated to surgery. The acute effects of IL-6 and other inflammatory mediators released during surgical trespass may contribute to postoperative anemia.[25,26] Although postoperative anemia would seem to be merely a matter of surgical blood loss, postoperative anemia will develop after procedures without significant blood loss. Anemia was observed to develop and persist at 1 month after bunionectomy among patients with an average intraoperative blood loss of 4 ml.[25] Moreover, the development of postoperative anemia without significant blood loss appears to be independent of the type of anesthesia administered.[26,27]
The acute inflammatory state caused by surgical manipulation of tissue may produce anemia similar to that produced by chronic inflammation. This may exacerbate anemia in patients with surgical blood loss by impairing the compensatory reticulocytosis to replace lost red blood cells.
Management: Reduced red blood cell mass may be treated by replacing cells (transfusion) or supporting the ability of the body to produce new cells. The potential benefit of transfusion must be weighed against the potential risks including transfusion reaction, transmission of infectious disease, transfusion-related acute lung injury, and immunosuppression.
In a retrospective study of 125 women undergoing surgery but refusing blood transfusion owing to religious objections, increased mortality was seen with postoperative hemoglobin less than 8 g/dl, with a sharply increased mortality below 6 g/dl.[28] Significant co-morbidity (cardiac, renal, hepatic) was a very poor prognostic sign in conjunction with severe postoperative anemia. The American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion recommends that red blood cells should usually be administered to healthy adults if the hemoglobin is less than 6 g/dl and are not indicated if the hemoglobin concentration is more than 10 g/dl. For wide and clinically significant hemoglobin range of 6–10 g/dl, transfusion should be based on potential or ongoing bleeding, intravascular volume status, and risk factors for complications of inadequate oxygenation or poor organ perfusion.[29] The ultimate goal for treatment of anemia is to provide adequate oxygenation to vital organs, reduce cardiac and cerebral hypoxic damage while protecting the patient from potential harm from transfusions. No single transfusion trigger based upon hemoglobin or hematocrit values achieves this goal for all patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree