Key Clinical Questions
What are the clinical and pharmacologic effects of single agent versus multiple agent sedative and analgesic administration?
What is the best way to dose sedative, analgesic, and paralytic agents in the intensive care unit (ICU)?
How do the pharmacokinetics and dosing of sedative, analgesic, and paralytic agents change with duration of infusion?
What is the proper sequence to initiate sedatives, analgesics, and/or paralytics, and how does dosing refinement affect time to weaning and other predictors of favorable outcome?
How should a hospitalist select individual sedative, analgesic, or paralytic agents in critically ill patients?
When should a hospitalist decide to paralyze a ventilated patient?
Introduction
The appropriate delivery of critical care to patients in the intensive care unit (ICU) requires a judicious use of sedative and analgesic regimens for many patients as well as the occasional need for neuromuscular blockade. Providers utilize various sedative-analgesic strategies to accomplish this goal, and pharmacologically induced paralysis may be undertaken in upwards of 10% of specific ICU populations. While management of the discomfort of mechanical ventilation serves as the primary indication for most sedative and analgesic regimens in the ICU, the increased myocardial oxygen consumption as well as immunosuppression resulting from catechol upregulation also represent important untoward outcomes to aggressively manage by treating pain and anxiety with analgesic and sedative medications.
Critically ill patients are subject to adverse stimuli that arise not only from endotracheal support apparatus, but also from indwelling monitoring lines and catheters and from surgical and other wounds. Sleep deprivation and encroachments on personal modesty also provoke further intolerance of the ICU environment. The significant discomforts of the ICU setting may be palliated with the use of sedative and analgesic therapy.
Sedative, analgesic, and paralytic therapies are administered by intravenous (IV) bolus or by IV infusion, as intramuscular and enteral titration are unreliable.
Bolus dosing has the advantage of a rapid onset of action, but has the associated risk of either over- or undershooting the therapeutic goal. As such, nursing vigilance with frequent assessment and bolus titration to defined therapeutic outcomes is necessary. On the other hand, infusion drug dosing reflects a more gradual and refined titration to clinical effect, but does carry a greater risk of drug accumulation with protracted infusion. As such, an unwanted prolongation of time to awakening may occur when clinical circumstances allow for drug discontinuation. For this reason, infusions should only be undertaken in the ICU when bolus dosing has failed to produce the desired sedative effect.
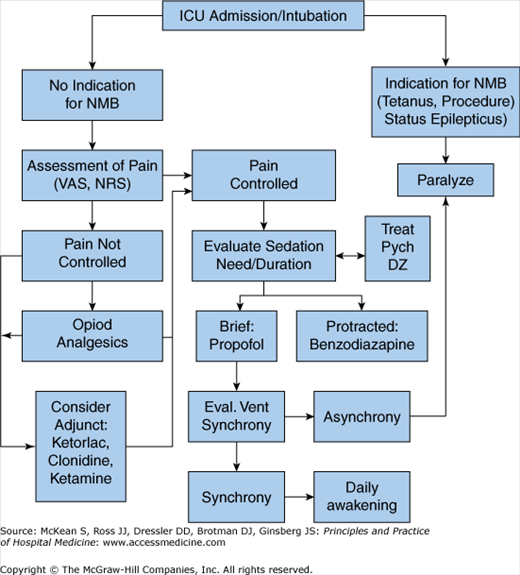
Clinicians should follow a structured algorithm for ICU sedation, pain control, and paralysis (Figure 135-1). While sedative and analgesic agents help to maintain mechanical ventilation their prolonged use can become an obstacle to liberation from life support. Because planning for weaning should occur as soon as ventilatory support is begun, the sedative and analgesic goals should be defined and dosing plan structured for each individual agent such that their sum individual dosing requirements are minimized.
Analgesic goals should be addressed first. Once adequate analgesia is achieved, sedation can be targeted to the patient’s clinical need. For instance, a patient with acute respiratory distress syndrome (ARDS) ventilated with a high-frequency oscillator may require deep sedation and analgesia (and possibly neuromuscular blockade), whereas a patient requiring minimal volume ventilation or pressure support may need only mild analgesia and sedation. An initial targeted focus on the patient’s analgesic need will help curtail sedative requirements, as adequate pain control decreases anxiety and sedative need.
Recent clinical evidence supports that sedation need not be applied as a routine aspect of ICU care even in the most severely ill intubated patients provided that adequate bolus analgesia (morphine) is carefully administered. Patients treated in this manner demonstrated shorter ICU stays and fewer ventilator days without suffering any untoward clinical outcomes. It may be reasonable to consider analgesia only in some patients and utilize sedation only for “rescue” purposes for patients intolerant of a purely analgesic regimen.
During the initiation phase of sedation and analgesia, the patient’s chronic comorbid conditions must be considered in guiding analgesic requirement. For instance, the patient may have a source of chronic pain such as musculoskeletal pain or neuropathy. This could be an overlooked source of discomfort if not factored in the appraisal of a patient’s analgesic need. Once pain and anxiety are controlled, sedation can also be reduced as tolerated. Similarly, preexisting psychiatric disorders such as psychosis should be taken into account in targeting sedation and anxiolysis.
Dose or infusion maintenance: Analgesic and sedative titration and active management should continue throughout the ICU stay. If the patient’s dyssynchrony with the ventilator is primarily a manifestation of untreated pain, continued escalation in dosing with sedative medications (eg, propofol) could lead to unnecessary hemodynamic compromise and drug accumulation. Alternately, adding a medication or switching to a medication with intrinsic analgesic properties may better benefit some patients.
Dose or infusion weaning and discontinuation: Withdrawal of sedative and analgesic medications may result in the development of delirium that may impede liberation from mechanical ventilation and unnecessary diagnostic workup. This type of delirium is common, having been reported in upwards of one-third of patients who have been sedated in the ICU for greater than a week.
A sensitivity to the signs of withdrawal from opioids (diaphoresis, tachycardia, papillary dilatation, lacrimation, piloerection, hypertension, vomiting, abdominal cramping, restlessness, anxiety, fever, yawning) and bezodiazapines (tremor, diaphoresis, anxiety, tachycardia, nausea) should be maintained. Some advocate a 10% decrement in narcotic use every day, but a tailored approach may be appropriate for some patients.
Caring for critically ill patients represents an extremely dynamic practice in which drug regimens with rapid clinical effects and short durations of action are frequently best suited. In general, drugs without active metabolites and with few pharmacologic interactions offer the greatest efficacy with the fewest side effects. Furthermore, medications with smaller volumes of distribution are more easily titrated. Drugs that do not manifest significant tachyphylaxis also lend themselves to titration in ICU practice. Sedatives and analgesics with increased risk of hemodynamic side effects are avoided when possible, as are medications with higher risk of withdrawal symptoms upon their discontinuation.
Table 135-1 contains a list of the pharmacologic characteristics of the ideal drug. While no single drug possesses every one of these ideal properties, sedative and analgesic medications are selected based on these parameters and the individual patient’s clinical condition. ICU patients can have depressed or changing hepatic and renal clearance profiles, as well as dynamic hepatic synthetic function and nutritional flux. Drug concentrations and protein binding can change as well. Similarly, alterations in gut absorption affect serum levels of enterally administered agents. Each patient’s prevailing physiology as well as the response to sedative and analgesic therapy should impact the titration of sedative, analgesic, and paralytic medications in the ICU.
| Clinical onset | Rapid |
| Clinical effect | Short |
| Active metabolites | None |
| Pharmacologic interactions | None |
| Volume of distribution | Small |
| Tachyphylaxis | None |
| Hemodynamic side effects | None |
| Withdrawal symptoms | None |
Drugs do not attain an immediate steady state, as they are influenced by the volume of distribution (Vd) within the patient and the variable redistribution into different anatomic compartments. Upon administration, drugs are preferentially distributed to vessel-rich organ systems. Drugs with lipophilic biochemical properties (eg, fentanyl, midazolam) are more rapidly distributed to the lipid-rich regions of the body, which includes the central nervous system (CNS) (Figure 135-2). Hydrophilic drugs (eg, morphine) on the other hand penetrate fatty tissue relatively poorly and are confined more to the lower Vd that is the plasma, with slower CNS penetration and clinical effect.
An administered drug will undergo renal and hepatic metabolism, but certain highly lipophilic drugs with relatively long elimination half-times by kidney and liver distribute rapidly at first into the vessel-rich group (including CNS), and then redistribute into a second “compartment” (plasma), thereby rapidly terminating their clinical (sedative) effect. With time and ongoing infusion, this termination of clinical effect by redistribution becomes attenuated as a new steady state is established between compartments (Figure 135-3). Broadly, these phenomena of variable pharmacokinetics contribute to the context-sensitive half-time of an agent. This principle represents a clinically useful way to understand how greater length of infusion prolongs clinical effect. Classical ways of thinking about drug elimination half-lives after bolus dosing by renal and hepatic metabolism have little clinical utility with ongoing infusion and changing distribution between compartments. The concept of context-sensitive half-time describes changing time to elimination of half of the current plasma concentration with discontinuation of a drug at that point in time. These dynamic pharmacokinetics have important implications in the ICU setting. Suitable arousal for ventilator weaning is very much dependant on CNS concentration of sedative agents at that particular point in time, which in turn is reliant on the context-sensitive half-time of the drug. With passing time, and with continued administration, lipophilic drugs that have come to a steady state within lipid rich compartments may redistribute systemically and manifest a declining dose requirement and a longer time to clinical awakening.
Concomitant use of sedative and analgesic agents will affect the properties of each of the drugs. Drugs of different pharmacologic classes may have synergistic or additive sedative or analgesic effects. A synergistic combination magnifies the relative clinical effect of each drug to a greater extent than would be predicted based on its individual dose potency, whereas an additive combination finds a clinical effect that approximates each drug’s individual relative potency. Benzodiazepine sedatives and opiate analgesic agents display synergism in analgesia, sedation, and respiratory depression. Therefore, more pronounced clinical effects occur when these two classes of drugs are used in combination than when each is used individually at higher doses. Because of this, unpredictable clinical effects can occur when drugs are coadministered, and combining these classes of medications should be done with caution in the non-ventilated or weaning patient.
Similarly, changing individual doses of drugs in a combination regimen should be done with the same care as the administration of a new drug because an entirely new pharmacodynamic state is established with differing combination drug concentrations. Drugs that are not primarily considered sedative agents, but nonetheless have sedating properties (eg, anticholinergic agents or neuroleptics), should also be used with cognizance of their potential additive or synergistic sedative capability. Finally, unlike the benzodiazepines, many commonly used sedative medications, such as propofol, have no individual analgesic properties or potentiation when used in combination with opioids.
Sedation in the ICU
The benzodiazepines and propofol represent the most commonly used agents for sedation and anxiolysis in critically ill patients. Both classes of drugs potentiate GABA inhibition within the CNS with anxiolytic, sedative, and hypnotic effects. Both have similar amnestic potential, which may be desirable within the ICU setting, and both have similar respiratory depressant effects due to shifting of the carbon dioxide (CO2) response curve rightward (decreased respiratory drive at physiologic CO2 levels) and blunting of hypoxic drive.
The individual drugs in both classes are lipid soluble. However, propofol is carried in a lipid emulsion that renders it much more lipophilic, which in turn accounts for the very rapid uptake into the lipid-rich and highly vascular CNS with subsequent quick redistribution out of the CNS after bolus dosing or brief infusions. This redistribution terminates propofol’s clinical sedative effect. Like all lipophilic agents, propofol and benzodiazepines will demonstrate a prolongation of clinical effect once they have been discontinued if their protracted use has resulted in saturation of the body’s “lipid compartment”.
The choice of whether to use a benzodiazepine or propofol depends on pharmocokinetic considerations and the matching of the time needed for sedation in a particular clinical circumstance to the pharmacokinetic properties of the drug. Propofol is more often the agent of choice for sedation for brief procedural interventions or short-term mechanical ventilation (less than one to three days), whereas the benzodiazapines might serve as a more cost-effective choice for sedation that is expected to last more than a few days. Based on the pharmacologic properties and important clinical considerations of the commonly used sedatives, benzodiazepines also often serve as the preferred agents for sedation when refractory hypotension is a concern, as they do not have the same degree of direct vasodilation that can occur with propofol.
Pharmacologic properties of commonly used benzodiazepine agents vary (Table 135-2) as do dosing regimens and costs of both benzodiazepine and opioid drugs (Table 135-3). The pharmacokinetic properties and dosing of sedative and analgesic agents were derived from studies of healthy volunteers who have received single bolus drug doses. These data may not accurately reflect dynamic context-sensitive half-times with continuous infusion, especially in critically ill patients with shifting volumes of distribution, serum drug-protein binding, severely compromised hepatic perfusion, and metabolism changes.
| Opioid Medications | Equianalgesic IV Dose | Intermittent Dosing | Infusion Dose | Onset (minutes) | Half-Life (hours) | Lipophilicity | Active Metabolites |
|---|---|---|---|---|---|---|---|
| Fentanyl | 100–200 mcg | 0.35–0.5 mcg/kg every 0.5–1 hour | 0.7–10.0 mcg/kg/hour | Immediate | 1.5–6 | +++ | Yes |
| Morphine | 10 mg | 0.01–0.15 mg/kg every 1–2 hours | 0.07–0.5 mg/kg/hour | < 5 | 3–7 | + | Yes |
| Hydromorphone | 1.5 mg | 10–30 mcg/kg every 1–2 hours | 7–15 mcg/kg/hour | < 5 | 2–3 | ++ | No |
| Meperidine | 70–100 mg | Not recommended | Not recommended | < 5 | 3–4 | +++ | Yes |
| Methadone | 10 mg | 10 mg every 6 hours | Not recommended | 10–20 | 15–30 | +++ | No |
Midazolam and lorazepam are the most commonly used benzodiazepines in the ICU. Although midazolam is thought of as a rapid-onset, short-acting agent, when used in the short term its high lipid solubility results in accumulation with prolonged infusion. This characteristic, along with the fact that its primary metabolite (1-hydroxymidazolam glucuronide) is clinically active for up to 36 hours after drug cessation, makes lorazepam a preferred sedative especially in the obese and in patients requiring greater than 48 hours of sedation.
|
Benzodiazepine withdrawal can occur with weaning from these drugs. Longer-acting agents such as diazepam or lorazepam may be used as a bridge to complete discontinuation of sedation.
Patients receiving greater than 50 mg total dose or greater than 1 mg/kg of IV lorazepam per day are at risk for toxicity from propylene glycol, a solvent in which the drug is dissolved. Propylene glycol toxicity can cause renal injury and acute tubular necrosis (ATN), tissue necrosis, and significant anion-gap acidosis. Patients receiving greater than the recommended daily dose of this drug should be monitored for toxicity with daily osmolar gap measurements.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree