KEY POINTS
Thoughtful clinical decision making often contributes more to the patient’s outcome than dramatic and innovative interventions or cutting-edge technology.
While protocols and checklists inform general care of patient populations in the ICU, for individual patients it is equally important to formulate clinical hypotheses based on an understanding of pathophysiology, then test them.
Define therapeutic goals and seek the least intensive intervention that achieves each.
Novel treatments require objective clinical trials before they are implemented, and traditional therapies require clarification of goals and adverse effects in each patient before their use can be optimized.
Determine daily whether the appropriate therapeutic goal is treatment for cure or treatment for palliation.
Critical care is invigorated by a scholarly approach, involving teaching, learning, and performing research.
INTRODUCTION
Intensive care has its roots in the resuscitation of dying patients. Exemplary critical care provides rapid therapeutic responses to failure of vital organ systems, utilizing standardized and effective protocols such as advanced cardiac life support and advanced trauma life support. Other critically ill patients in less urgent need of resuscitation remain vulnerable to multiple organ system failure, and benefit from prevention or titrated care of each organ system dysfunction according to principles for ultimately reestablishing normal physiology. This critical care tempo differs from the time-honored rounding and prescription practiced by most internists and primary care physicians. Furthermore, the critical care physicians providing resuscitation and titrated care often have little firsthand familiarity with their patients’ chronic health history, but extraordinary tools for noninvasive and invasive description and correction of their current pathophysiology. Though well prepared for providing cure of the acute life-threatening problems, the intensivist is frequently tasked with the responsibility of being the bearer of bad news when recovery is impossible, and must regularly use compassionate pastoral skills to help comfort dying patients and their significant others, using clinical judgment to help them decide to forego further life-sustaining treatment. Accordingly, experienced intensivists develop ways to curb their inclination toward action in order to minimize complications of critical care, while organizing the delivery of critical care to integrate and coordinate the efforts of many team members to help minimize the intrinsic tendency toward fragmented care. In academic critical care units, teaching and investigation of critical care are energized by the clinical practice; in turn, the practice is informed, animated, and balanced by the information and environment arising from and around teaching and research programs. Yet the vast majority of critical care is delivered in community-based ICUs not affiliated with universities,1,2 where critical care physicians rely on their penchant for lifelong learning to update their knowledge and skills through informed reading and participating in continuing medical education. These activities provide a means for all critical care physicians to maintain career-long learning and access to new understandings of the management of critical illness.
PROVIDING EXEMPLARY CARE
Clinical excellence is founded in careful history taking, physical examination, and laboratory testing. These data serve to raise questions concerning the mechanisms for the patient’s disease, on which a complete prioritized differential diagnosis is formulated and treatment plan initiated. The reality, complexity, and limitations apparent daily in the ICU present several pitfalls on the path to exemplary practice. By its very nature, critical care is exciting and attracts physicians having an inclination toward action. Despite its obvious utility in urgent circumstances, this proclivity can replace effective clinical discipline with excessive unfocused ICU procedures. This common approach inverts the stable pyramid of bedside skills, placing most attention on the least informative source of data, while losing the rational foundation for diagnosis and treatment.
An associated problem is that ICU procedures become an end in themselves rather than a means to answer thoughtful clinical questions. Too often these procedures are implemented to provide monitoring, ignoring the fact that the only alarm resides in the intensivist’s intellect. Students of critical care benefit from the dictum: “Don’t just do something, stand there.” Take the time to process the gathered data to formulate a working hypothesis concerning the mechanisms responsible for each patient’s main problems, so that the next diagnostic or treatment intervention can best test that possibility. Without this thoughtful clinical decision making, students of critical care are swept away by the burgeoning armamentarium of the ICU toward the unproductive subspecialty of critical care technology. So often in the ICU thoughtful compilation of the patient’s health evaluation preceding the acute event is more helpful than acquiring new data defining the current pathophysiologic state. Accordingly, attention to this search for meaningful collateral history and the retrieval of prior radiologic studies and laboratory values often should precede the next invasive ICU procedure. The next intervention should be chosen to test a diagnostic hypothesis formulated by thoughtful processing of the available data.
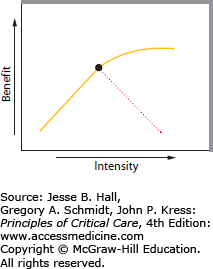
Testing a therapeutic hypothesis requires knowing the goal of the intervention and titrating the therapy toward that end point. Too often clinicians managing initial care employ too little too late during resuscitation. For example, physicians unfamiliar with the pace and treatment of hypovolemic shock may order a bolus of 250 mL of crystalloid solution followed by 200 mL/h, while the mean blood pressure rises from 50 to 60 mm Hg over 2 hours. A far better volume resuscitation protocol targets urgent restoration of a normal blood pressure and perfusion, so a bolus of a liter is given every 10 to 20 minutes, to continue until the blood pressure exceeds 90 mm Hg without inducing pulmonary edema. Similarly the results of recent trials of approaches to treating septic shock are consistent with a view that more important than placement of invasive monitoring devices and adhering to complex treatment algorithms is the administration of appropriate antibiotics and adequate fluid volumes promptly after the development of hypotension.3-5 Evidence from other clinical trials informs us that interventions such as fluid resuscitation should not be open ended but used only to the point of adequate resuscitation, since adverse effects of excessive fluid administration are likely.6,7 This principle of titration of therapy toward a thoughtful end point without causing common adverse effects is depicted in Figure 1-1.
FIGURE 1-1.
A schematic diagram relating therapeutic intensity (abscissa) to the benefit of therapy (ordinate). For many interventions in critical illness, there is a monotonic increase in benefit as treatment intensity increases (solid line), but concomitant adverse effects of the intervention cause harm at higher intensity (interrupted line) (for examples, see text). This leads to an approach to critical care that defines the overall goal of each intervention and seeks the least intense means of achieving it.
One of the consequences of protocol-driven resuscitations is that the recovered patient now has more treatments than diagnoses. An effective approach to the adverse outcome of excess therapeutic interventions is the mindset that liberates the patient from these potentially harmful interventions as rapidly as their removal is tolerated. For example, the patient with hemorrhagic shock treated with volume resuscitation and blood products also acutely receives intravenous vasoconstricting agents to maintain perfusion pressure while hemostasis and volume resuscitation are achieved. Once a stable blood pressure and hemostasis are achieved, what is the time course for discontinuing catecholamines?
One answer is to wean the vasoconstrictor slowly (eg, decrease the norepinephrine infusion rate from 30 by 5 μg/min each hour). Another approach is to liberate the patient from the vasoconstrictor by reducing the norepinephrine infusion rate by half every 15 minutes. The difference between these two approaches is more than the time taken to discontinue the agent, for if in the second approach the blood pressure were to fall after reducing the norepinephrine to 15 μg/min, the critical care physician learns that the patient remains hypovolemic and needs more volume infusion; the first approach would mask the hypovolemic hypoperfused state by the prolonged use of vasoconstrictor agents, leading to the adverse consequences of multiple organ hypoperfusion. Words convey meaning, and to wean connotes the removal of a nurturing, even friendly life-support system from a dependent, deprived infant, a process that should proceed slowly; by contrast, liberation is the removal of an unnecessary and potentially toxic intervention from an otherwise independent adult, a process that should proceed urgently.8 Similarly, other aspects of critical care management as simple as bed rest and sedative administration are best approached as treatments from which the patient should be liberated at the earliest opportunity.9,10
Thus the principle that “less is more” applies to many critical care therapies including bed rest, fluid therapy, vasoactive drug use, mechanical ventilation, and administration of sedative and muscle-relaxing agents. Of course, the difficulty in all these examples is that the therapeutic intervention is initially necessary and/or lifesaving, but how long the intervention needs to continue for the patient’s benefit versus the patient’s harm depends on a critical evaluation of the goal of therapy.
Figure 1-1 indicates the intensity-benefit relationship of many of these interventions (eg, the continued use of high-dose norepinephrine in the hypotensive patient with hemorrhagic shock discussed earlier). During the initial resuscitation, the benefit of increasing the norepinephrine dose along the x-axis (intensity) was demonstrated by the rising blood pressure during hemostasis, volume resuscitation, and norepinephrine infusion. Yet blood pressure is not the appropriate benefit sought in the hypoperfused patient, but rather adequate perfusion of all organs. Even without measuring cardiac output, an adequate perfusion state could be inferred from an adequate blood pressure when the vasoconstrictor agent is diminished. However, with continued infusion of the vasoconstrictor, the adverse effect of a prolonged hypoperfusion state, even with an adequate blood pressure, is indicated by the interrupted line, which illustrates a decreasing benefit as the intensity of the intervention and the shock it masks continues. Armed with this rationale, the intensivist should progressively reduce the intensity of norepinephrine infusion over a relatively short period to determine whether the volume resuscitation is adequate.
A second example is the use of fluid restriction and diuresis in the treatment of pulmonary edema. In Figure 1-1 the intensity of the intervention is the achievement of negative fluid balance while the benefit would be the reduction of pulmonary edema. Considerable data suggest a monotonic relationship between the intensity of these therapeutic interventions and the benefit of reduced pulmonary edema.11 Yet, if intravascular volume is reduced too much, there is a consequent reduction in the cardiac output, so the benefit to the patient is more than offset by the attendant hypoperfusion state. The thoughtful intensivist recognizes that the goal of reducing pulmonary edema should not induce a hypoperfusion state, so the targeted intensity is the lowest intravascular volume associated with an adequate cardiac output and oxygen delivery to the peripheral tissues.
Beyond enhancing the clinical scholarship of critical care, this approach maximizes another hallowed principle of patient care—“First do no harm.” Despite excited opinions to the contrary, effective critical care is rarely based on brilliant, incisive, dramatic, and innovative interventions, but most often derives from meticulously identifying and titrating each of the patient’s multiple problems toward improvements at an urgent but continuous pace. This conservative approach breeds skepticism toward innovative strategies: Novel treatments require objective clinical trials before they are implemented, and traditional therapies require clarification of goals and adverse effects in each patient before their use can be optimized.12-14 Accordingly, intensivists should carefully consider the experimental support for each diagnostic and therapeutic approach to critical illness and acknowledge that each approach has adverse effects in order to define the least intensive intervention required to achieve its stated therapeutic goal.
The ICU has long evolved beyond a room in which ventilators are used. Instead, in a well-functioning ICU, the physical plant and technology are planned to facilitate the delivery of care, while also responding to new opportunities in this rapidly evolving field. The physician director, the nurse manager, and the team of respiratory therapists, pharmacists, and physiotherapists must build a mutually supportive environment conducive to teaching, learning, and care.
Intensivists must be aware of the economic and legal concerns as ICUs capture the interest of politicians, ethicists, and the courts. Furthermore, the managers of ICUs should build on experience. Quality assurance, triage and severity scoring, and infection surveillance are essential to the continued smooth running of ICUs and indeed to their improvement over time.
MANAGING DEATH AND DYING IN THE INTENSIVE CARE UNIT
Perhaps no critical care issue is more emotionally charged and time-consuming than the decision to withhold and/or withdraw life-sustaining therapy. Practitioners and students of critical care are frequently called on to guide patients and their families through this complex decision-making process. Accordingly, we discuss an approach to managing death and dying in the ICU meant to minimize one current adverse outcome of modern critical care—our patients die alone in pain and distress because maximal care aimed at cure proceeds despite little chance of success.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree