FIGURE 1 Effects of ongoing nociception (e.g. due to surgery) on central pain processing. There is progressive rostral neuraxial spread of central sensitisation, black-filled circles 1–3. Nerve damage results in altered CNS function, unfilled circles 1–4. Manifestations of CNS sensitisation are listed in the grey clouds. Rostral spread of central sensitisation is subject to descending modulation, black filled circles 4–5.
MEASURING ALTERED PAIN PROCESSING IN PATIENTS
With the advent of validated quantitative sensory testing (QST) methods suitable for use in patients it has become feasible to research the alterations in pain processing following surgery in clinical patient cohorts [13,14,25,27,53–56]. Reliable QST techniques are now available to assess the time course of both aspects of altered central pain processing named above, namely spreading central sensitisation and dysfunction of inhibitory descending pain modulation. Databases documenting pain sensitivities in healthy volunteers are now available, permitting assessment of individual degree of deviation from normal values [38,39]. We have developed and implemented a clinical screening QST paradigm suitable for this purpose [50]. The Nijmegen-Aalborg Screening QST (NASQ) paradigm detects spreading central sensitisation by bilaterally mapping pain sensitivity at several standardised body sites [50]. Heterotopic spread of sensitisation is assessed by measuring skin as well as deep tissue pain sensitivity using electric and pressure algometry devices. The NASQ investigates dysfunction of descending endogenous pain modulation using a conditioned pain modulation (CPM) paradigm [20,37,59]. The NASQ CPM paradigm uses the cold pressor task as conditioning stimulation, and electric and pressure pain tolerance thresholds as test stimuli before and after conditioning stimulation. As an alternative QST approach, other groups have implemented mapping techniques, e.g. using pinprick stimulation, to detect areas of hyperalgesia surrounding sites of surgery [13,14,25,27,43].
Using such QST paradigms, we are now in a position to explore the alterations in central pain processing resulting from the ongoing nociception of surgery, their relationship to chronic pain development after surgery, and the factors influencing post-surgical altered pain processing and chronic pain.
EARLY POSTOPERATIVE PERIOD: PAIN PROCESSING IN THE FIRST WEEK AFTER SURGERY
A number of studies have followed alterations in central pain processing using pain thresholds in the first week after surgery [53,55,56]. Figure 2 shows a typical example from this first type of studies [56].
Taken together, such studies show a biphasic response in the first week after surgery regarding pain sensitivity. In the first 24 hours postoperatively there is a generalised, predominantly hypoalgesic response, more marked caudally. This is followed by hyperalgesia both close to – and distant from – surgery five days after the operation. Postoperative alterations in pain processing do not correlate well with clinical pain measures such as VAS or morphine patient-controlled analgesia use. Pre- and intraoperative analgesic supplementation (e.g. opioids, ketamine, magnesium) increases hypoalgesia in the first 24 hours after surgery, particularly distant from surgery. It also markedly decreases hyperalgesia distant from surgery five days postoperatively, with smaller effects on peri-incisional hyperalgesia. This positive effect is not seen with ketorolac supplementation. The marked day five hyperalgesia seen after back surgery is not seen after abdominal hysterectomy, probably due to the absence of the extensive nerve damage seen in back pain and surgery. Of interest is the fact that the presence of pain preoperatively in back surgery patients is linked to much more – and increasing – hyperalgesia after surgery, particularly at five days (Fig. 3).
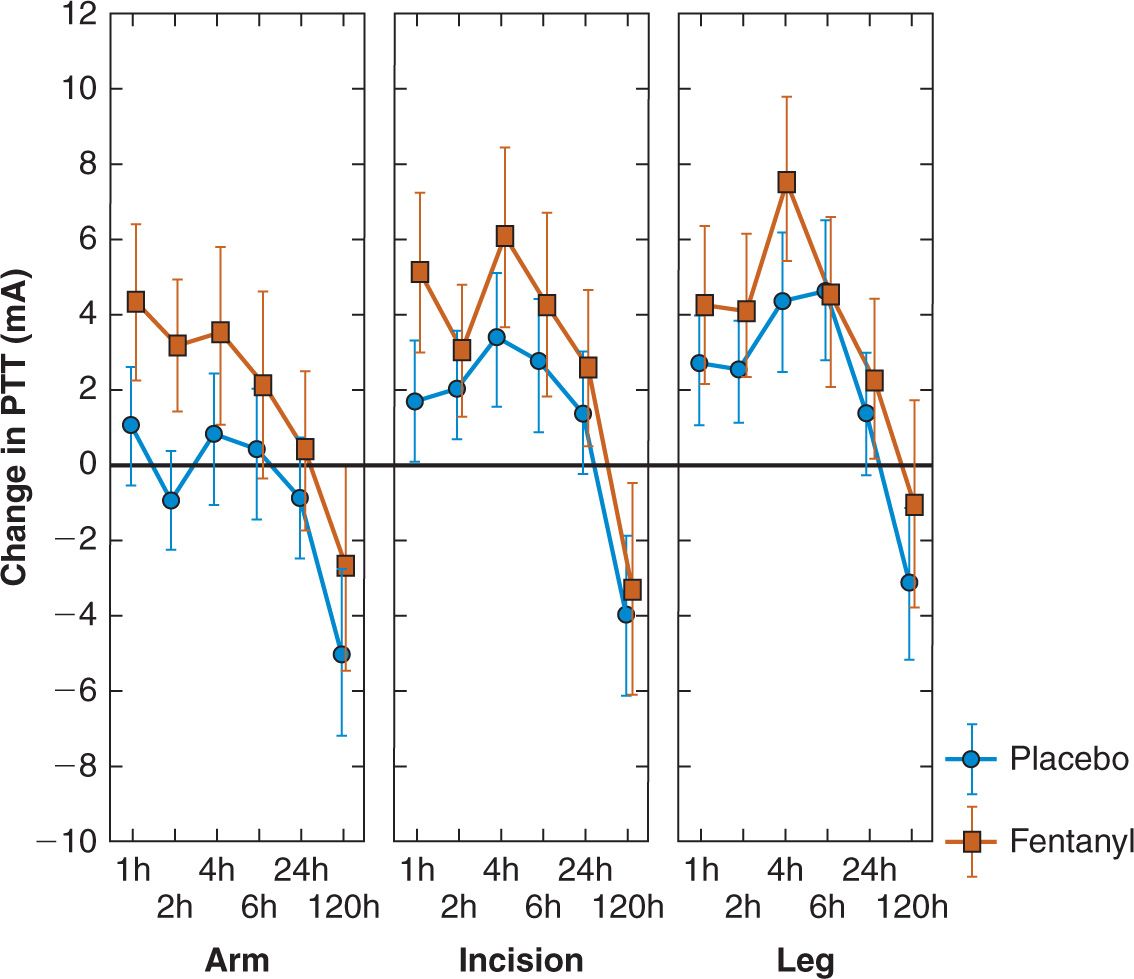
FIGURE 2 Alterations in electric pain tolerance thresholds in patients after back surgery. They show a predominantly hypoalgesic response, greater in the presence of analgesic opioid (fentanyl) supplementation, for the first 24 hours postoperatively. Five days postoperatively, significant segmental and spreading hyperalgesia appears, predominantly in the placebo group. The vertical axis shows change in electric pain tolerance thresholds vs. preoperatively (in mA). The upper horizontal axis shows time postoperatively in hours; the lower horizontal axis shows site of threshold measurement (arm, peri-incisionally in the back, leg). Values are means and 95% confidence intervals. (Modified from reference Wilder-Smith OH, Tassonyi E, Senly C, et al. Surgical pain is followed not only by spinal sensitization but also by supraspinal antinociception. Br J Anaesth 1996;76(6):816–821).
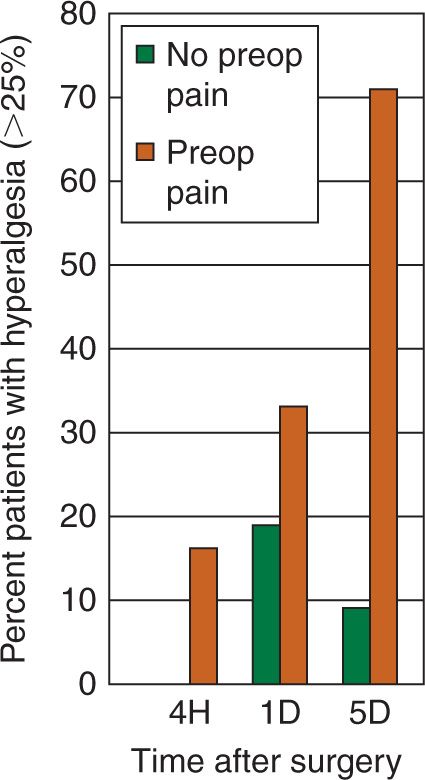
FIGURE 3 Effects of presence or absence of preoperative pain on incidence (in percent) of postoperative hyperalgesia greater than 25% (vertical axis) in back surgery patients. Horizontal axis gives hours (H) or days (D) postoperatively. (Modified after reference Wilder-Smith OH. Chronic pain and surgery: a review of new insights from sensory testing. J Pain Palliat Care Pharmacother 2011;25(2):146–159).
A second type of study uses pinprick stimulation to map early peri-incisional changes in pain processing [13,14,25,27,43]. An example of such a study is summarised in Figure 4 [25]. The findings are congruent with those of the first type of studies. Again there is no strong link between postoperative hyperalgesia and clinical measures of pain. The area of peri-incisional hyperalgesia increases with time the first days after surgery, and perioperative analgesic supplementation or locoregional analgesia use are associated with smaller areas of postoperative peri-incisional hyperalgesia. Of particular importance is the demonstration of a strong link between greater areas of early (week one) postoperative peri-incisional hyperalgesia and more chronic pain up to one year after surgery [14,25,27]. Moreover, nerve damage is not only associated with larger areas of hyperalgesia but also with more chronic pain [27].
In summary, pain processing during the first postoperative week initially shifts in the direction of acute, generalised inhibition (the first day or so). This is followed by the development and rapid spread of hyperalgesia not only over the skin but also to deeper tissues. Worse perioperative analgesia and nerve damage are associated with more week 1 spreading hyperalgesia; this in turn is associated with more persisting pain up to one year postoperatively. Subjective pain experience (e.g. pain scores) does not reliably reflect these alterations in central pain processing.
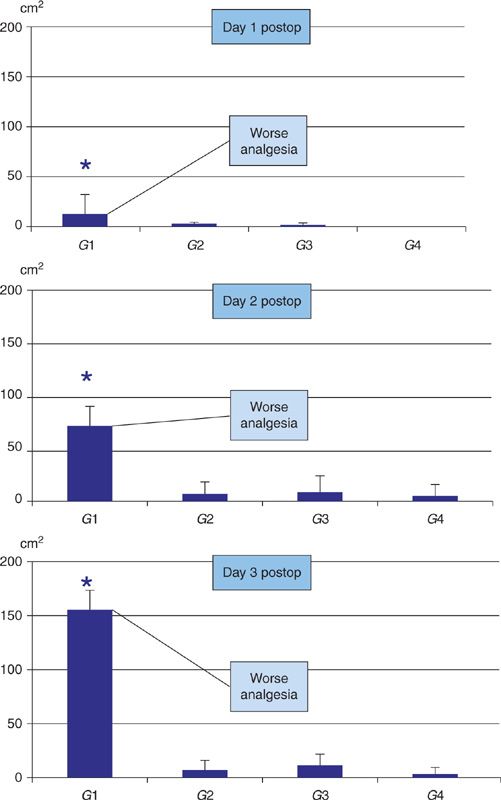
FIGURE 4 Area (vertical axis) of secondary mechanical hyperalgesia surrounding abdominal incision for colectomy measured using von Frey filaments at 24, 48, and 72 h postop. At all times hyperalgesic area was larger in group 1 than in groups 2–4 (* P < 0.05). Grouping was according to intra/postoperative analgesia Group 1: IV–IV (systemic analgesia); Group 2: IV–epidural, Group 3: epidural–epidural, Group 4: epidural–IV. (Modified after reference Lavand’homme P, De Kock M, Waterloos H. Intraoperative epidural analgesia combined with ketamine provides effective preventive analgesia in patients undergoing major digestive surgery. Anesthesiology 2005;103(4):813–820).
LATE POSTOPERATIVE PERIOD: PAIN PROCESSING UP TO SIX MONTHS AFTER SURGERY
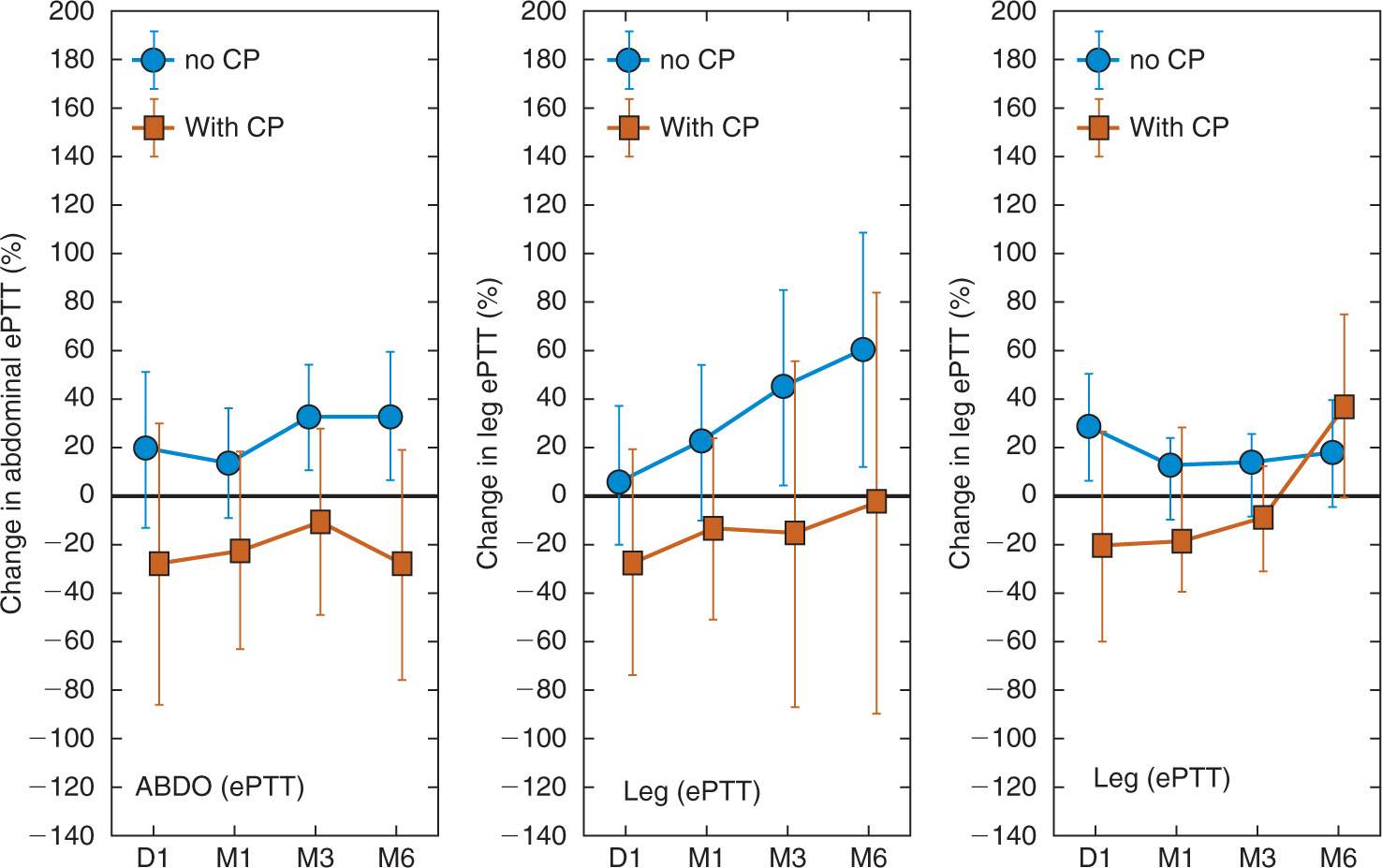
We are aware of only one study prospectively, systematically, and longitudinally documenting the long-term time course of alterations in central pain processing after surgery [54]. The main results of this study recruiting patients undergoing major abdominal surgery in standardised general anaesthesia are summarised in Figure 5. Compared to patients not reporting chronic pain, patients with chronic pain show significantly more skin and deep tissue hyperalgesia distant from the surgical site one day to six months after surgery. Patients with chronic pain also exhibit more peri-incisional skin hyperalgesia in the six month postoperative time course. As with the studies covering the early postoperative period, the differences in central pain processing up to three months postoperatively are also not well-reflected in clinical pain measures. The prominent presence and role of hyperalgesia in the development of chronic pain after surgery is supported by a number of cross-sectional studies documenting excitatory alterations of pain processing in patients reporting chronic pain after surgery [5,19,28,29], thus also confirming that chronic pain after surgery shares mechanisms with other types of chronic pain regarding altered central pain processing.
In summary, early evidence suggests that patients who manifest persistent or chronic pain up to six months after surgery show increased and marked spread of sensitisation (expressed as spreading hyperalgesia) in their central nervous system. This spread is both rostral (towards supraspinal structures) and heterotopic (to other, deeper tissues). To date, no studies are available regarding effects of therapeutic interventions on this process. Again, subjective pain experience poorly reflects these alterations in central pain processing.