Key Clinical Questions
What are the predisposing diseases/conditions that lead to acute respiratory distress syndrome (ARDS)?
How can ARDS be differentiated from other causes of hypoxemic respiratory failure?
What is the correct strategy in the management of mechanical ventilation in ARDS?
What adjunctive therapies may be beneficial in ARDS management?
What is the expected morbidity for patients who survive ARDS to hospital discharge?
Introduction
The acute respiratory distress syndrome (ARDS) describes a common disorder encountered in the critical care unit that remains a significant cause of morbidity and mortality since its initial description in 1967. Standardization of ventilator strategies and overall improvement in critical care management, however, has resulted in significant improvements in outcomes in the last decade. Early recognition is essential in order to admit and/or transfer the patient to a higher level of care, when indicated.
Epidemiology
Since the publication of the American-European Consensus Conference (AECC) (see Diagnosis) definitions for acute lung injury (ALI) and ARDS, the incidence has been identified in several studies. Without differentiating between ALI and ARDS, the incidence may range from 20 to 50 cases per 100,000 person-years. When rigorous screening for ALI and ARDS was applied in King County, Washington in 2000, investigators reported an incidence of 78.9 cases/100,000 person-years and 58.7 cases/100,000 person-years, respectively. Methodological differences may explain the disparity in incidence, as some reports retrospectively review prior ARDS studies while others prospectively identify patients admitted to an intensive care unit (ICU) or among those requiring mechanical ventilation. Inaccuracies in true incidence are magnified by a nonspecific case definition—the AECC ARDS criteria—and the relative lack of validation studies for said criteria (see Diagnosis).
Presentation
Patients presenting with acute lung injury or impending ARDS may be difficult to differentiate initially from other causes of hypoxemic respiratory failure. A predisposing cause of ALI/ARDS should be present, with pulmonary and non-pulmonary sepsis most common (Table 134-1). Knowledge of the common etiologies of ARDS is important as early vigilance in regards to moving the patient to a higher level of care (eg, transferring to the ICU) and initiation of appropriate ICU interventions can minimize morbidity and mortality. Furthermore, identification of an underlying cause or inciting event is important in differentiating ARDS from other lung diseases or syndromes that may be misidentified as ARDS. Early and efficient identification of predisposing syndromes may prevent progression to ALI or ARDS. The evolution from inciting cause to ARDS typically occurs within 3 to 5 days, giving the clinician a window of suspicion for ARDS to develop.
| Direct Lung Injury | Indirect Lung Injury |
|---|---|
| Pneumonia | Nonpulmonary sepsis |
| Gastric aspiration | Acute pancreatitis |
| Chest trauma/lung contusion | Nonchest trauma |
| Inhalation injury | Massive transfusions |
| Near-drowning | Surface burns |
|
The presenting complaint may be nonspecific and herald a more serious problem which accompanies the predisposing condition. Shortness of breath, dyspnea, and cough are likely present. Fever and sputum production may indicate a pulmonary infection, while hypoxemia requiring high fractions of inspired supplemental oxygen suggests evolving acute lung injury with worsening ventilation-perfusion (V/Q) mismatching and worsening shunt physiology. The evaluating clinician may describe declining level of consciousness secondary to severe hypoxemia or concomitant hypercapnia. Concerning findings such as altered mentation and hypercapnia usually warrant immediate endotracheal intubation. Tachypnea and auscultation of rales on physical examination is typical of ALI/ARDS but may also present in other causes of acute respiratory failure such as congestive heart failure, pneumonia, or occult interstitial lung disease. Laboratory studies are not specific for ARDS. Severe hypoxemia is part of the diagnostic criteria of ALI and ARDS. Concomitant hypercapnia may indicate failure of compensatory ventilatory mechanisms. Failure to compensate for a respiratory acidosis or inadequate clearance of carbon dioxide in the face of tachypnea is especially worrisome and warrants immediate attention. Leukocytosis, also nonspecific, may belie the predisposing cause as in severe pneumonia or sepsis.
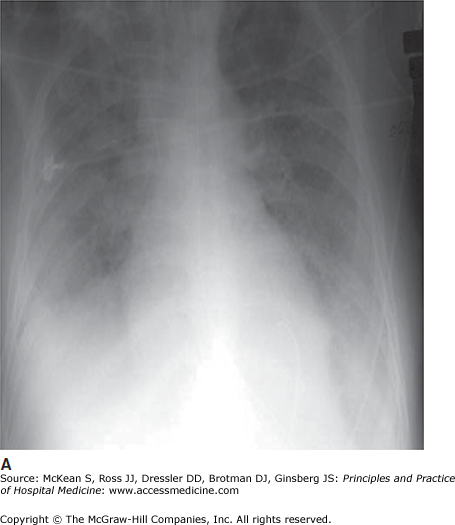
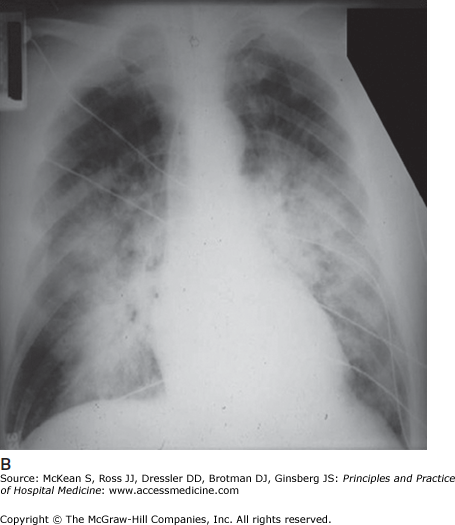
Chest radiography reveals bilateral, diffuse airspace infiltrates. Patchy infiltrates may become more confluent as the syndrome evolves (Figure 134-1). Cardiomegaly suggests preexisting cardiac disease and thus left ventricular dysfunction as an etiology for the patient’s hypoxemia and chest radiograph abnormality. In cases where there is concern about coexistent or predominant heart failure, additional testing (echocardiogram or pulmonary artery catheter, for example) may be required to confirm the diagnosis of ARDS or rule out other conditions.
Pathophysiology
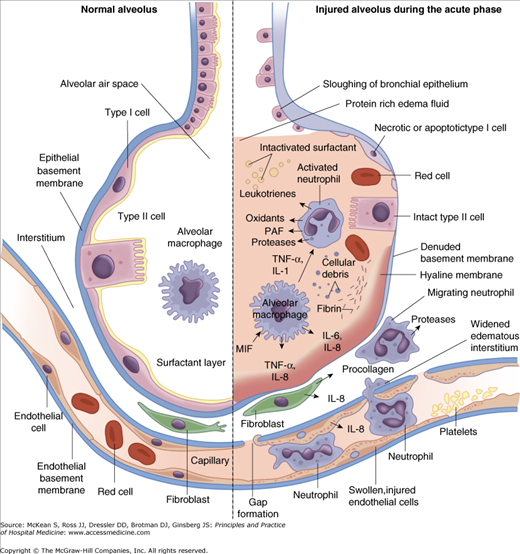
Integrity of the alveolar-capillary membrane is required to maintain a dry alveolar space and to ensure adequate gas exchange between the alveolar epithelium across the interstitial space to the pulmonary capillaries. The predisposing injury leading to ARDS results in several pathophysiological changes at every level of the alveolar-capillary compartment (Figure 134-2).
The alveolar epithelium is composed primarily of two types of cells. Type I pneumocytes function primarily in architectural support as well as fluid and solute transport through its aquaporin-5 surface proteins. The larger, but fewer, type II pneumocytes are capable of differentiating into type I cells. These cells primarily produce and recycle surfactant. The type I cell is more susceptible to injury, and during the early phases of ARDS (the acute, exudative phase) barrier integrity is disrupted as type I cells are damaged. Dilution of edema fluid in the alveolus and decreased production following type II cell damage impairs surfactant function. This contributes to atelectasis and worsening pulmonary compliance.
Similar injury occurs on the capillary endothelial side of the compartment. In ALI/ARDS, the alveolar-capillary barrier loses its ability to limit egress of fluids, proteins, and debris from the vascular space. The combination of vascular hydrostatic and protein osmotic pressures, together with vascular integrity, sets the stage for accumulation of proteinaceous pulmonary edema according to Starling’s equation, first described in 1896:
The Starling equation states that movement of fluid across a capillary barrier into an interstitial space is governed by balances in the hydrostatic pressure in the capillary and interstitium (Pc and Pi, respectively) and the plasma and interstitial oncotic pressures (πpp and πip, respectively). Increases in capillary hydrostatic pressure or decreases capillary oncotic pressure will result in net movement of fluid out of the plasma space and in to the interstitial subcompartment. The capillary reflection coefficient (σ) and the bulk transfer coefficient (Kf) describe the integrity of the capillary barrier: as s approaches zero the barrier loses its ability to segregate oncotically active substances, while increases in Kf linearly increase movement of fluid across the membrane. The injury seen early in acute lung injury facilitates movement of fluid out of the capillary compartment by a combination of these changes.
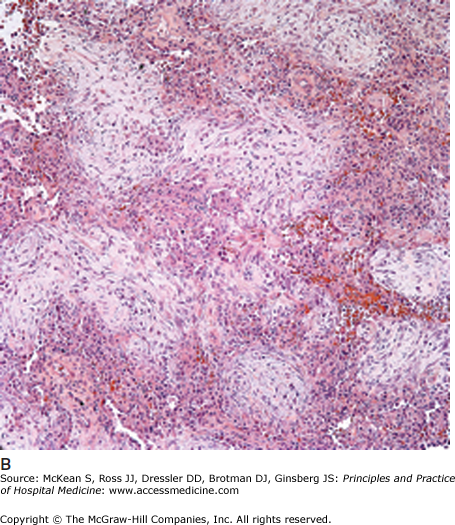
In this early stage of ARDS, shunting and hypoxemia dominate the clinical picture. The flooding of the alveolar unit with proteinaceous Edema leads to worsening V/Q mismatching and physiologic shunt that is increasingly refractory to oxygen supplementation. The atelectasis caused by surfactant defects requires higher pressures to maintain alveolar patency for ventilation. Increases in pulmonary artery pressures secondary to hypoxemic pulmonary vasoconstriction, decreases in pulmonary circulation due to microthrombi and direct damage to the vascular endothelium lead to worsening dead space ventilation. Together, each of these contributes to increased work of breathing. The histological change seen in the exudative phase is known as diffuse alveolar damage (Figure 134-3A). Nonspecific for ARDS, diffuse alveolar damage is not considered diagnostic and a lung biopsy is not necessary unless other alternative diagnoses are seriously considered.
Figure 134-3
(A) Exudative phase, diffuse alveolar damage: Prominent eosinophilic hyaline membranes line the alveolar ducts and sacs. (B) Proliferative phase, organizing diffuse alveolar damage: Extensive young fibroblastic proliferation fills the alveolar spaces and interstitium. (Courtesy of Anthony A. Gal, MD.)
|
These changes in the exudative phase of ARDS are heterogeneous and of variable duration, typically lasting 7 to 10 days. Following the exudative phase, the lungs progress to the second stage of disease known as the proliferative phase (Figure 134-3B). Type II pneumocytes begin to regenerate in order to reconstitute the surfactant layer, and type I cells rebuild the damaged alveolar epithelium. Depending on the effectiveness of this reparative process, recovery from ARDS in days 7 through 21 may be rapid. Some patients, however, have a prolonged course as procollagen III is deposited in the interstitial space with subsequent fibrosis. A dominant or exaggerated fibrotic phase increases the risk for prolonged morbidity or mortality and may require prolonged mechanical ventilation. As the vascular changes that occur in the exudative phase become more irreversible, pulmonary hypertension may result.