Guillain-Barré syndrome (GBS) is the major cause of acute flaccid paralysis. Its annual incidence in children under 9 years of age is 0.62 per 100,000.
Guillain-Barré syndrome (GBS) is the major cause of acute flaccid paralysis. Its annual incidence in children under 9 years of age is 0.62 per 100,000. GBS characteristically presents as a progressive, ascending, symmetric, areflexic weakness of more than one limb, with autonomic dysfunction.
GBS characteristically presents as a progressive, ascending, symmetric, areflexic weakness of more than one limb, with autonomic dysfunction. The proportion of various subtypes of GBS varies with the geographical regions. Acute inflammatory demyelinating polyradiculopathy accounts for 85%-90% of cases of GBS in Europe and North America.
The proportion of various subtypes of GBS varies with the geographical regions. Acute inflammatory demyelinating polyradiculopathy accounts for 85%-90% of cases of GBS in Europe and North America. The diagnosis is based on a characteristic clinical picture, nerve-conduction abnormalities, and the cerebrospinal fluid, which shows dissociation in albumin level and cell count.
The diagnosis is based on a characteristic clinical picture, nerve-conduction abnormalities, and the cerebrospinal fluid, which shows dissociation in albumin level and cell count. Indications for PICU admission include rapid progression of motor weakness involving respiratory muscles, ventilatory insufficiency, pneumonia, severe bulbar weakness, autonomic instability, arrhythmia, or bradycardia. Autonomic dysfunction is an important cause of death due to hemodynamic instability and arrhythmias.
Indications for PICU admission include rapid progression of motor weakness involving respiratory muscles, ventilatory insufficiency, pneumonia, severe bulbar weakness, autonomic instability, arrhythmia, or bradycardia. Autonomic dysfunction is an important cause of death due to hemodynamic instability and arrhythmias. Autonomic instability and risk for succinylcholine-induced hyperkalemia/arrhythmia complicate the intubation of these patients.
Autonomic instability and risk for succinylcholine-induced hyperkalemia/arrhythmia complicate the intubation of these patients. Treatment involves either plasma exchange (50 mL/kg) or intravenous immunoglobulin (IVIG) 400 mg/kg/day for 5 days. IVIG may be preferable for young children whose line access for plasmapheresis is likely to be difficult.
Treatment involves either plasma exchange (50 mL/kg) or intravenous immunoglobulin (IVIG) 400 mg/kg/day for 5 days. IVIG may be preferable for young children whose line access for plasmapheresis is likely to be difficult. Plasmapheresis may be preferable for (a) patients with immunoglobulin A (IgA) deficiency who would be at risk for anaphylaxis with IVIG administration, (b) patients with congestive heart failure, or (c) those at risk of volume overload.
Plasmapheresis may be preferable for (a) patients with immunoglobulin A (IgA) deficiency who would be at risk for anaphylaxis with IVIG administration, (b) patients with congestive heart failure, or (c) those at risk of volume overload. Myasthenia gravis (MG) is characterized by fluctuating weakness and fatigability, especially of ocular muscles.
Myasthenia gravis (MG) is characterized by fluctuating weakness and fatigability, especially of ocular muscles. Diagnosis of MG is made with positive Tensilon/Neostigmine test, acetylcholine receptor antibodies (in 85% of cases), decremental response to repetitive nerve stimulation, and abnormal single-fiber electromyogram.
Diagnosis of MG is made with positive Tensilon/Neostigmine test, acetylcholine receptor antibodies (in 85% of cases), decremental response to repetitive nerve stimulation, and abnormal single-fiber electromyogram. Myasthenic crisis, defined as respiratory failure (which occurs in 15%-20% patients), must be differentiated from cholinergic crisis.
Myasthenic crisis, defined as respiratory failure (which occurs in 15%-20% patients), must be differentiated from cholinergic crisis. The porphyrias comprise a group of disorders caused by enzymatic defects in heme the biosynthesis that leads to an overproduction and accumulation of 5-aminolevulinic acid and porphobilinogen.
The porphyrias comprise a group of disorders caused by enzymatic defects in heme the biosynthesis that leads to an overproduction and accumulation of 5-aminolevulinic acid and porphobilinogen. Clinical features of acute pophyria include a triad of abdominal pain, changes in mental state, and peripheral neuropathy; respiratory paralysis and failure occur in up to 20% patients.
Clinical features of acute pophyria include a triad of abdominal pain, changes in mental state, and peripheral neuropathy; respiratory paralysis and failure occur in up to 20% patients. Precipitating factors of acute intermittent porphyria are drugs, starvation, hormonal factors, and infections.
Precipitating factors of acute intermittent porphyria are drugs, starvation, hormonal factors, and infections. Treatment of an acute attack includes stopping offending drug(s), control of, pain using opiate analgesics, hypertension using propranolol, seizures using diazepam, and gastrointestinal symptoms using promethazine. Specific therapy is IV 10% glucose and hemin infusion (3-4 mg/kg, once daily for 4 days). One needs to be alert to the increased risk of hyponatremia with use of large volumes of 10% glucose.
Treatment of an acute attack includes stopping offending drug(s), control of, pain using opiate analgesics, hypertension using propranolol, seizures using diazepam, and gastrointestinal symptoms using promethazine. Specific therapy is IV 10% glucose and hemin infusion (3-4 mg/kg, once daily for 4 days). One needs to be alert to the increased risk of hyponatremia with use of large volumes of 10% glucose. In adults, intensive care unit-acquired weakness is common and adds to hospital length of stay and cost of care.
In adults, intensive care unit-acquired weakness is common and adds to hospital length of stay and cost of care. The disorder is broadly categorized into three subcategories: critical illness polyneuropathy (CIP), critical illness myopathy (CIM), and critical illness neuromyopathy.
The disorder is broadly categorized into three subcategories: critical illness polyneuropathy (CIP), critical illness myopathy (CIM), and critical illness neuromyopathy. No specific treatments are recommended. Strict glycemic control using intensive insulin therapy has been shown to significantly reduce the incidence of CIP/CIM in adults.
No specific treatments are recommended. Strict glycemic control using intensive insulin therapy has been shown to significantly reduce the incidence of CIP/CIM in adults. Diaphragmatic palsy can be bilateral or unilateral. Unilateral palsy is more common in infants and children. Most common causes are birth trauma and cardiac surgery.
Diaphragmatic palsy can be bilateral or unilateral. Unilateral palsy is more common in infants and children. Most common causes are birth trauma and cardiac surgery. Unilateral palsy is often asymptomatic in older children, but in newborn infants and young children, it can cause severe respiratory compromise.
Unilateral palsy is often asymptomatic in older children, but in newborn infants and young children, it can cause severe respiratory compromise. Fluoroscopy on sniffing is a traditional method of diagnosis. Sonographic assessment of diaphragmatic motion can be used for diagnosis and to follow the progression.
Fluoroscopy on sniffing is a traditional method of diagnosis. Sonographic assessment of diaphragmatic motion can be used for diagnosis and to follow the progression. Surgical plication is accepted as the effective treatment. Diaphragmatic palsy secondary to phrenic nerve injury in the newborn invariably requires surgery. Older children, if asymptomatic, may be managed conservatively.
Surgical plication is accepted as the effective treatment. Diaphragmatic palsy secondary to phrenic nerve injury in the newborn invariably requires surgery. Older children, if asymptomatic, may be managed conservatively.TABLE 53.1 ANATOMIC CLASSIFICATION AND EXAMPLES OF CONDITIONS WITH NEUROMUSCULAR WEAKNESS THAT REQUIRE PEDIATRIC INTENSIVE CARE | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
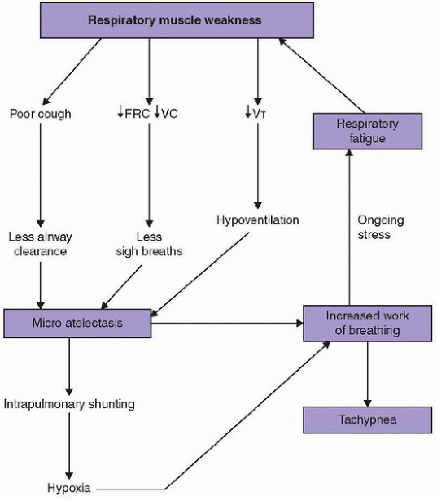
for respiratory support have all been correlated with significant reductions in clinical parameters.
TABLE 53.2 CLINICAL AND LABORATORY PARAMETERS THAT ARE USEFUL IN ASSESSMENT OF ADEQUATE RESPIRATORY FUNCTION IN PATIENTS WITH NEUROMUSCULAR WEAKNESS | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
condition. Respiratory status, therefore, must be closely and carefully monitored.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree