Acute Coronary Syndromes
Coauthored by Shailesh Shetty
KEY POINTS
1 In ischemic heart disease, survival and ventricular function are maximized by rapidly reestablishing sufficient myocardial blood flow to prevent myocardial necrosis. percutaneous coronary intervention is the reperfusion modality of choice, but if there are substantial delays in transfer then fibrinolytic therapy should be used in lytic eligible patients. Remember that the door-to-balloon time should be less than 90 min.
2 Reducing myocardial oxygen consumption by limiting heart rate (avoidance of exercise and use of β-blockade), reducing afterload (controlling hypertension and normalizing ventricular filling pressures), and reducing contractility (alleviation of excessive catecholamine stimulation and use of β-blockers) are important steps to optimize myocardial supply and demand.
3 Myocardial oxygen supply can be quickly and simply boosted with nitrates, supplemental oxygen, and optimization of hemoglobin concentration.
4 Most patients should receive agents to interrupt the clotting cascade, as well as oxygen, pain relievers, and β-blockers. All suitable candidates should be considered for immediate interventional procedures (angioplasty/stent) or for antithrombotic therapy. The presence of ST segment elevation and the duration of chest pain prior to arrival have a direct bearing on the value of thrombolytics and interventional catheterization.
5 Early administration of an angiotensin converting enzyme inhibitor should be considered to reduce infarct expansion and minimize the risk of ventricular dysfunction.
▪ NON-ST ELEVATION ACUTE CORONARY SYNDROMES: UNSTABLE ANGINA AND NON-ST ELEVATION MYOCARDIAL INFARCTION
Definitions and Pathophysiology of Acute Coronary Syndrome
Unstable angina (UA) and non-ST segment elevation myocardial infarction (NSTEMI) are now grouped under the heading of non-ST elevation acute coronary syndromes (NSTE-ACSs). Because they share a common underlying pathophysiology, the management of these two conditions is quite similar. UA is synonymous with the terms preinfarction angina, crescendo angina, intermediate coronary syndrome, and acute coronary insufficiency. NSTEMI implies non-Q wave myocardial injury. The main difference between UA and NSTEMI is that biomarkers of myocardial necrosis are elevated in the latter (e.g., creatine kinase-myocardial band [CK-MB], troponin-I, troponin-T).
Myocardial ischemia results from an imbalance between oxygen supply and demand. Anginal chest pain is the clinical expression of this imbalance. Because the left ventricle (LV) comprises most of the cardiac muscle mass and faces the greater afterload, it is at higher risk for ischemia. Myocardial oxygen delivery may be limited by (a) coronary atherosclerosis, (b) plaque rupture with thrombosis, (c) coronary artery spasm, (d) anemia, (e) hypoxemia, (f) limited diastolic filling time (tachycardia), and (g) hypotension.
Four major factors increase cardiac oxygen demand: (a) tachycardia and/or increased systemic metabolic demands for cardiac output; (b) heightened LV afterload causing increased transmural
wall tension (e.g., hypertension, LV cavity dilatation, aortic stenosis); (c) increased LV mass (hypertrophy); and (d) increased contractility. Despite the predisposition of the LV to ischemia, conditions that cause hypertrophy, dilatation, or increased afterloading of the right ventricle (RV) also can put its muscle mass at risk. For example, pulmonary embolism may precipitate RV ischemia—a phenomenon that is most common in patients with underlying right coronary artery (RCA) narrowing or cor pulmonale.
wall tension (e.g., hypertension, LV cavity dilatation, aortic stenosis); (c) increased LV mass (hypertrophy); and (d) increased contractility. Despite the predisposition of the LV to ischemia, conditions that cause hypertrophy, dilatation, or increased afterloading of the right ventricle (RV) also can put its muscle mass at risk. For example, pulmonary embolism may precipitate RV ischemia—a phenomenon that is most common in patients with underlying right coronary artery (RCA) narrowing or cor pulmonale.
An unstable coronary atherosclerotic plaque is the key to the pathophysiology of ACS. Histologic studies of coronary vessels have shown that atherosclerotic plaques are intimomedial in location. In general, there two types of coronary plaques: (a) stable plaque with small lipid core and thick fibrous cap and (b) unstable plaque with large lipid core and thin cap. The former generally causes stable angina pectoris if it causes significant obstruction of the vessel (>50% to 70% of the vessel lumen diameter). The bulky, soft, lipid-laden plaques are more prone to rupture and ACS. Many of these plaques do not cause significant obstruction of the lumen of coronary vessels before the onset of the ACS. Hence, the patient may not have experienced any cardiac symptoms prior to the onset of ACS.
Acute instability and rupture of one or more coronary plaques with superimposed thrombosis play a central role in the pathophysiology of ACS. This clot, composed of platelets and thrombin, not only produces a fixed vessel occlusion but also promotes reversible vasoconstriction. The resulting sudden coronary artery occlusion, which may be total or subtotal, causes acute myocardial ischemia or infarction. UA represents a high-risk transition period during which most patients undergo accelerated myocardial ischemia. If unchecked, this transition culminates in acute myocardial infarction (AMI) or sudden cardiac death (SCD) in up to 15% of patients within just a few weeks. Coronary angiography in many of these patients demonstrates complex coronary plaque lesions with varying degrees of superimposed thrombosis. Intravascular ultrasonic examination of coronary vessels (IVUS) is another useful tool that has helped shed considerable light, not only on the pathophysiology but also on the management of coronary artery disease, particularly in the setting of ACS.
The role of platelets in the pathophysiology of ACS has undergone considerable review in the past decade or so. Platelet activation and aggregation play a significant role in the formation and propagation of a platelet-rich or “white” clot over a ruptured atherosclerotic plaque in patients with UA and NSTEMI. This is contrast to the fibrin-rich or “red” clot seen in the coronaries of patients with STEMI. The current recommendations on the use of antithrombin and antiplatelet therapies in NSTEMI and that of fibrinolytic therapy in patients with STEMI derive not only from the pathophysiology of these conditions, but also from the results of various clinical trials performed within the last decade.
Diagnosis
History and Physical Examination
The term UA denotes new pain or a departure from a previous anginal pattern. UA occurs at rest or with less provocation than stable angina. Pain lasting longer than 15 min also suggests UA. Angina occurring in the early post-MI period or within weeks of an interventional coronary procedure also is best termed “unstable.” Commonly, the pain is described as a “tightness,” “heaviness,” or “squeezing” in the substernal region. UA may awaken patients from sleep or present as pain at a new site such as the jaw or arm. Autonomic manifestations (nausea, vomiting, or sweating) also favor “instability.” Blood pressure frequently rises before the onset of pain, even in resting patients. Rising blood pressure boosts afterload, increasing wall tension and myocardial O2 consumption. Less commonly, the onset of congestive heart failure (CHF) may be the only manifestation of UA.
Data Profile
Electrocardiographic Changes
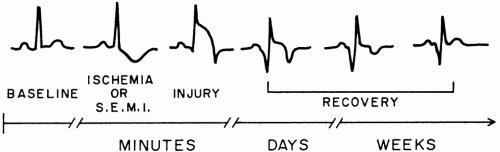
During episodes of ischemic chest pain, electrocardiogram (ECG) features may include (a) ST-segment elevation or depression, (b) T wave flattening or inversion, (c) premature ventricular contractions (PVCs), or (d) conduction disturbances, including bundle branch block (Fig. 21-1). Reversible ST depression or T wave inversion is seen in most patients if continuous ECG monitoring is used, a finding that may not emerge during a single 12-lead ECG. Even with intensive monitoring, ECG findings are absent in up to 15% of symptomatic patients with UA. Therefore, a normal ECG does not exclude a diagnosis of UA or MI. Conversely, up to 70% of all ECG-documented episodes of ischemia are clinically silent.
Cardiac Enzyme Markers
Elevated total CK (including the CK-MB fraction) and cardiac troponins (I and T) are markers of myocardial necrosis and indicate an MI, even in the absence of convincing ST segment-T wave changes. Troponins (I/T) are more sensitive and specific in making the diagnosis of an AMI than CK-MB. Troponin elevation in NSTE-ACS correlates with adverse prognosis. These are also patients who are likely to benefit from Glycoprotein 2b/3a (Gp2b/3a) receptor antagonist therapy and from early coronary angiography and revascularization. Highly sensitive C-reactive protein (hs-CRP) levels are also increased in patients with ACS. ACS patients with the highest levels of hs-CRP and troponins have the worst prognosis.
Prognostic Factors
Patients with UA have a lower short-term mortality rate (2% to 3% at 30 days) compared to those with acute NSTEMI (5% to 7% at 30 days). The in-hospital or short-term mortality of patients with STEMI is higher compared with those with NSTEMI (6% to 9% vs. 5% to 7% at 30 days). However, the longterm mortality in NSTEMI (10% to 12%) is similar to or greater than that associated with STEMI (9% to 11%), likely due to their greater incidence of multivessel coronary artery disease.
Thrombolysis in Myocardial Infarction Risk Score
Several risk variables have been identified in patients with NSTE-ACS. A value of 1 has been assigned to each risk variable, and the total score has been shown to bear a linear relationship with risk of adverse events (death, MI, recurrent ischemia, and need for urgent revascularization) in the short term. The variables are (a) age greater than or equal to 65 years, (b) prior coronary stenosis greater than or equal to 50%, (c) presence of greater than or equal to three coronary risk factors, (d) ST segment deviation on admission ECG, (e) elevated cardiac biomarkers, (f) greater than or equal to two anginal episodes in last 24 h, and (g) prior use of aspirin (marker for vascular disease). The adverse event rate is 4% to 5% for thrombolysis in myocardial infarction (TIMI) risk score of 0 to 1 but approaches 40% for those with score of 6 to 7. Elevated levels of hs-CRP indicate a worse prognosis in each TIMI scoring category.
Management of NSTE-ACS
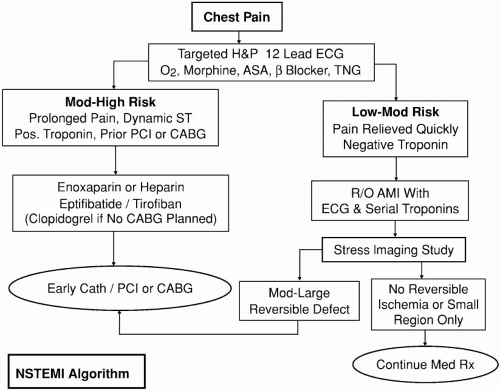
Patients with NSTE-ACS should be monitored closely and should receive aggressive antithrombotic, antiplatelet, and antianginal treatments (Fig. 21-2). Most patients with UA can be stabilized with appropriate medical therapy. Coronary angiography and revascularization procedures have become increasingly popular in these patients over the course of the last decade. Although emergent coronary angiography and revascularization procedures are uncommon in NSTE-ACS patients, most need to undergo coronary angiography and possible revascularization within a few days of admission to the hospital. Coronary revascularization procedures include either percutaneous coronary interventions (PCIs) (PTCA and stenting) or coronary artery bypass graft (CABG) surgery. Only patients with contraindications for invasive cardiac procedures are treated with noninvasive medical management. The two basic principles in the treatment of UA are to reduce myocardial O2 demand and improve O2 supply.
Reducing Myocardial Oxygen Consumption
The principal measures to decrease myocardial oxygen consumption are to limit heart rate and afterload. These goals are immediately accomplished by curtailing physical activity with bedrest. Exercise stress-tests are contraindicated in unstable patients since frank infarction may ensue. Arrhythmias like atrial fibrillation (AF) and ST should be controlled, both to reduce O2 consumption and to optimize diastolic filling time, thereby maximizing the sufficiency of coronary perfusion. Controlling hypertension and CHF decreases myocardial wall tension and therefore facilitates perfusion (see Chapter 22). Situations that increase heart rate (anxiety, use of short-acting nifedipine) or both heart rate and total body oxygen consumption (e.g., thyrotoxicosis, alcohol withdrawal, stimulant drug intoxication, anxiety, agitation, infections, etc.) should be promptly recognized and corrected. β-Blockers effectively reduce myocardial oxygen consumption by decreasing heart rate and cardiac contractility and improve O2 supply by lengthening diastolic filling time. β-Blocking drugs are particularly useful in reducing oxygen consumption in the tachycardic and hypertensive patient with UA but are contraindicated in acute heart failure, coronary artery spasm, or severe bronchospasm. β-Blocking agents also reduce risk of tachyarrhythmias in patients with ACS.
Increasing Myocardial Oxygen Supply
The most important treatment under this category are the strategies for myocardial revascularization which include percutaneous coronary angioplasty, coronary stenting, and coronary artery bypass surgery (will be dealt with later in this chapter). Myocardial oxygen supply can also be increased simply by boosting hemoglobin saturation or elevating hemoglobin concentration to levels higher than 9 or 10 gm/dL, in severely anemic patients.
Pharmacotherapy is also necessary to optimize myocardial perfusion. Nitroglycerin (NTG) is used commonly and may be administered sublingually, orally, transcutaneously, or intravenously. (For unstable patients, the intravenous route is most reliable.) In addition to dilating coronary vessels, NTG also decreases wall tension of the LV by reducing preload and, to a lesser extent, afterload. Acting through these mechanisms, NTG also reduces the risks of life-threatening arrhythmias in acute ischemia. Nitrates are effective both for classical and variant angina because of their direct coronary vasodilating properties. NTG is titrated to relieve chest pain or to reduce blood pressure by 10% to 20%. Usually, intravenous doses of 0.7 to 2.0 μg/kg/min suffice. Intravenous NTG usually is begun at 5 to 15 μg/min and titrated upward as necessary in increments of 5 μg/min every 5 min up to a maximum dose of 200 μg/min. Headache is a common side effect but usually responds to simple oral analgesics. When the dose is excessive or the patient is dehydrated, hypotension and reflex tachycardia result from NTG-induced vasodilatation. These adverse effects usually can be offset by volume expansion or α-agonist therapy. Because ethanol is used as a vehicle for NTG infusions, violent adverse reactions may occur in patients taking Antabuse. Obviously, use of high doses of NTG for prolonged periods may also produce alcohol intoxication. Within 48 to 72 h of initiating NTG therapy, tolerance is often observed, necessitating higher
infusion rates. Rare problems induced by NTG therapy include increased intraocular and intracranial (IC) pressures and methemoglobinemia.
infusion rates. Rare problems induced by NTG therapy include increased intraocular and intracranial (IC) pressures and methemoglobinemia.
Coronary spasm, a major contributor to myocardial ischemia in certain settings, may be ameliorated by nitrates or calcium channel blockers. Blockers of slow calcium channels (e.g., nifedipine, nicardipine, and amlodipine) can be rapidly effective in reversing coronary spasm. In UA, these drugs should be viewed as adjuncts to nitrate, β-blocker, and antithrombotic therapy. Because calcium antagonists have vasodilating, negative inotropic, and positive chronotropic actions, they may have detrimental effects for certain patients. If coronary vasodilating effects predominate, the myocardial oxygen supply-demand balances benefits. Conversely, if systemic vasodilatation, hypotension, and reflex tachycardia predominate, myocardial oxygen demand can outstrip supply and ischemia can worsen. Therefore, caution must be exercised to avoid hypotension or excessive tachycardia when using calcium channel antagonists.
Antiplatelet Therapy
Aspirin
Most patients with NSTE-ACS have an ulcerated atherosclerotic plaque covered by a subocclusive accumulation of platelets, thrombin, and red blood cells. Typically, these patients have platelet-rich or “white-clot.” Therefore, aggressive antiplatelet therapies are effective in stabilizing patients with ACS. Aspirin (162 to 325 mg daily) should be initiated immediately for all patients with ACS unless compelling contraindications exist. Cyclooxygenase-1 (COX-1) mediated platelet aggregation is inhibited within 15 min of aspirin administration, if nonenteric coated tablets are chewed and swallowed. Aspirin reduces synthesis of both thromboxane A2 (TXA-2) as well as prostacyclin. TXA-2 is a powerful promoter of platelet aggregation. Prostacyclin, on the other hand, promotes vasodilatation and inhibits platelet aggregation. Low-dose aspirin preferentially inhibits TXA-2 synthesis, and endothelial prostacyclin synthesis is inhibited by high-dose aspirin. In the VA cooperative study, Canadian multicenter trial, and RISC trial, aspirin was found to reduce the risk of death and AMI by approximately 50% in patients with NSTE-ACS. In a large metaanalysis by the antithrombotic trialist collaboration, aspirin reduced risk of death, MI, and stroke by about 46%. The benefits of aspirin may persist for years with continued therapy. The risk of recurrent events is reduced by at least 25%. The risk of coronary reocclusion after PCIs is reduced by about 50% with use of aspirin. At the low doses (75 to 150 mg) needed for platelet inhibition, few hemorrhagic or gastrointestinal side effects occur. At a lower dose, aspirin caused 2.5% major bleeds with 1% requiring transfusions. Aspirin resistance is seen in about 5% to 10% of patients, and these individuals are at increased risk for cardiovascular events. Although inhibition of platelet aggregation may complicate subsequent coronary artery surgery, aspirin-related clotting defects are reversible with platelet transfusions. Dipyridamole does not enhance the protective effect of aspirin in coronary ischemia, but clopidogrel and ticlopidine do complement the anti-ischemic effect of aspirin.
Clopidogrel, Ticlopidine, and Prasugrel
These agents belong to the thienopyridine class. They prevent platelet aggregation by noncompetitive inhibition of the adenosine diphosphate (ADP) binding to the type 2 purinergic (P2Y12) receptor, thereby inhibiting the activation of the glycoprotein IIb/IIIa receptor complex. Ticlopidine requires 3 to 6 days of therapy for full antiplatelet effect and carries a small risk of neutropenia (2.5%) and thrombotic thrombocytopenic purpura-hemolytic uremia syndrome (TTP-HUS). TTP-HUS occurs in the frequency of 1 in 1,500 to 5,000 patients taking ticlopidine. Both these life-threatening complications are most often seen within the first 12 weeks of therapy and necessitate immediate withdrawal of the drug. Patients with TTP-HUS may also need plasmapheresis. The usual dosage of ticlopidine is 250 mg by mouth twice daily. Ticlopidine has largely been replaced by clopidogrel.
Clopidogrel has been extensively studied in patients with ACS and in those who have received intracoronary stents. The life-threatening adverse effects seen with ticlopidine are far fewer with clopidogrel. In the CURE trial, clopidogrel use in ACS was found to significantly reduce risk of cardiovascular events (mostly reinfarctions) compared to aspirin alone. The usefulness of clopidogrel as an agent in reducing risk of cardiovascular events in patients who have received coronary stents has been clearly demonstrated in the PCI-CURE and CREDO trials. Clopidogrel is usually given as an oral bolus of 300 mg, followed thereafter at a dose of 75 mg once daily. Unlike ticlopidine, the antiplatelet effects of clopidogrel are seen within hours. Significant blood levels may be achieved sooner with a larger bolus dose of the medication (600 or 1,200 mg).
Along with ASA (81 mg once daily), it is given for a month after the implantation of bare-metal coronary stents and for at least 3 to 6 months after insertion of drug-eluting coronary stents. In patients with ACS, clopidogrel can be continued for 9 to 12 months. In some individuals at high risk for future cardiovascular events, clopidogrel with lowdose aspirin may be continued indefinitely if there are no contraindications and if cost is not an issue. There is a slight but significant increase in risk of bleeding with combination of clopidogrel and aspirin (3% to 5% risk of major bleeding), particularly in the elderly population.
Along with ASA (81 mg once daily), it is given for a month after the implantation of bare-metal coronary stents and for at least 3 to 6 months after insertion of drug-eluting coronary stents. In patients with ACS, clopidogrel can be continued for 9 to 12 months. In some individuals at high risk for future cardiovascular events, clopidogrel with lowdose aspirin may be continued indefinitely if there are no contraindications and if cost is not an issue. There is a slight but significant increase in risk of bleeding with combination of clopidogrel and aspirin (3% to 5% risk of major bleeding), particularly in the elderly population.
Prasugrel is the latest of the oral thienopyridine ADP-receptor antagonists available for patients with ACS. It is more a powerful antiplatelet agent as compared with clopidogrel and ticlopidine.
The TRITON-TIMI 38 study compared combination of prasugrel and aspirin to combination of clopidogrel and aspirin in patients with ACS undergoing percutneous coronary intervention. There were over 13,000 patients in this study and the primary endpoint was reduction of cardiovascular death, nonfatal stroke and nonfatal MI over 6 to 15 month follow-up period. The major safety endpoint was major bleeding. There was a significant reduction in primary endpoints with prasugrel compared with clopidogrel, including significant reduction in death, target vessel revascularization, and stent thrombosis. The risk of stent thrombosis was reduced by nearly over 50%. However, there was a significant increase in risk of serious bleeding (both fatal and nonfatal), which somewhat offsets the benefits. This drug is not yet being routinely used, but is likely to become more popular as the role for drug-eluting stents (DES) begins to expand. This may be a good agent for those who present with stent thrombosis with clopidogrel, in those with multiple DES, and in those at less risk of bleeding (like younger patient population).
Glycoprotein 2b/3a Receptor Inhibitors
Gp2b/3a receptor inhibitor agents are the most powerful intravenous form of antiplatelet agents available. The Gp2b/3a receptor binds to fibrinogen, which actually forms the molecular link that bridges adjacent platelets in the process of platelet aggregation. By binding to the Gp2b/3a receptors, these agents inhibit binding of fibrinogen to this receptor and thus inhibit platelet aggregation. The use of these agents in the management of ACS has increased significantly in the last 8 to 10 years, with a positive impact on patient outcomes. There are two broad classes of these agents: (a) large-molecule agents like abciximab (ReoPro) and (b) small molecule agents (peptidelike eptifibatide [Integrilin] and non-peptide-like tirofiban [Aggrastat]). Because abciximab molecules bind irreversibly to the Gp2b/3a receptor and produce permanent noncompetitive platelet inhibition, the clinical effects of the medication can last for 7 to 10 days. Severe uncontrolled bleeding associated with abciximab should be addressed by stopping the medication and transfusing platelets. The small molecule agents bind reversibly to the Gp2b/3a receptor to produce competitive platelet inhibition. The antiplatelet effects usually reverse within 4 to 6 h of stopping the medication. Platelet transfusions should not be given for bleeding with small-molecule Gp2b/3a receptor antagonists, as their presence also inhibits new platelet formation.
There have been a number of studies that have proven the efficacy of these agents in reducing the risk of cardiovascular events (death, recurrent ischemia, MI) in patients with ACS. Their beneficial effects have also been proven in the setting of PCIs, both in the patients with ACS and in those with stable coronary artery disease undergoing elective PCI procedures. Typically, small-molecule agents like tirofiban or eptifibatide are used along with aspirin, heparin, and other usual medications for initial stabilization of patients with NSTE-ACS. Tirofiban and eptifibatide are given as a bolus, followed by infusion of the drug lasting for 24 to 72 h. These agents are most beneficial in reducing cardiovascular events of those ACS patients at greatest risk, like those with ST-T abnormalities on ECG, elevated troponins, diabetes mellitus, and those with higher TIMI risk scores. The maximum benefit from use of Gp2b/3a receptor antagonists is seen in those individuals who undergo PCI during their hospital stay. The agents are of questionable benefit in those at low risk (lower TIMI risk score) and those who do not undergo PCI. The majority of high-risk patients undergo coronary angiography and PCI while receiving these agents. The drug infusions are usually continued for 12 to 24 h after the coronary intervention. If patients need coronary bypass surgery, then the medication infusion is stopped for at least 4 to 6 h before proceeding with surgery to minimize risk of bleeding.
Abciximab, on the other hand, is the preferred Gp2b/3a receptor antagonist during PCI in patients with STEMI. However, most centers use the small-molecular weight agents even in patients with STEMI. Abciximab intravenous infusion is
continued for 12 h after PCI. If patients on abciximab need emergent bypass surgery, then the medication is stopped and the patients are given a 6- to 12-unit platelet transfusion before coming off the cardiopulmonary bypass pump.
continued for 12 h after PCI. If patients on abciximab need emergent bypass surgery, then the medication is stopped and the patients are given a 6- to 12-unit platelet transfusion before coming off the cardiopulmonary bypass pump.
The risk of major bleeding with Gp2b/3a receptor antagonists is 2.5% to 4.0%. Most of the bleeding experienced from these agents is from vascular access sites after PCI. Severe thrombocytopenia with counts less than 50,000/mm3 is seen in 0.5% to 1.5% of patients who receive abciximab. Because thrombocytopenia can develop within hours of initiating an abciximab infusion, it is prudent to check platelet counts within 4 h of starting the infusion and again at the end of the infusion. Severe thrombocytopenia is rare with small-molecule agents (tirofiban and eptifibatide). There is also a small chance (0.5% to 1.0%) of developing serious pulmonary hemorrhage with abciximab therapy. This is potentially fatal condition and is rarely if ever encountered with the small-molecular weight Gp2b/3a receptor antagonists. The reasons have made the small-molecular weight agents popular with the cardiologists.
Antithrombotic Therapy
Unfractionated Heparin
Adequate doses of intravenous heparin given urgently along with oral aspirin reduce mortality and morbidity in patients with ACS severalfold by immediately interrupting the process of clotting on the coronary endothelium. The combination of heparin and aspirin is superior to aspirin alone in preventing the early complications of UA. Superiority of the combination probably results from the different mechanisms of the two treatments: heparin inhibits soluble clotting factors and thrombin-mediated platelet aggregation, whereas aspirin inhibits COX-mediated platelet aggregation. Even though the addition of heparin to aspirin raises the bleeding incidence slightly, the risk-benefit ratio almost always favors combination therapy. The goal of heparin therapy is to rapidly achieve and maintain a partial thromboplastin time (PTT) of 1.5 to 2.0 times the patient’s baseline or laboratory control value. This goal is best achieved using an intravenous bolus (60 units/kg, with a maximum dose of 4,000 units), followed by a continuous intravenous heparin infusion at a rate of 12 units/kg/h (maximum 1,000 units/h). The heparin infusion should be continued until coronary revascularization. Today, most of the ACS patients receive an intravenous infusion of a Gp2b/3a receptor antagonist for 12 to 24 h after PCI. They are also typically on aspirin and clopidogrel long term after PCI. In patients who are candidates for coronary bypass surgery, heparin and aspirin should be continued until surgery. In patients who are not candidates for coronary angiography and revascularization, heparin should be continued for 3 to 5 days. There is a risk of rebound angina when the heparin infusion is stopped. Thereafter, long-term use of aspirin alone can result in a 50% reduction in the incidence of angina recurrence.
Unfractionated heparin (UFH) is a heterogenous mixture of polysaccharides with molecular weights ranging from 3,000 to 30,000. There are several disadvantages with UFH. The antithrombin binding sites of heparin can be bound by a number of other plasma proteins, by platelet factor 4 and also by endothelial cells, thereby diminishing its therapeutic effect. Furthermore, heparin does not bind to clot-bound thrombin and to factor-Xa bound to platelets inside a clot. Thus, there is the possibility of clot propagation while the patient is receiving heparin. Heparin-induced thrombocytopenia (HIT) is another serious adverse effect.
Low-Molecular-Weight Heparins
These are homogenous glycosaminoglycans with molecular weight ranging from 4,000 to 6,000. Low-molecular-weight heparins (LMWH) have greater anti-factor Xa activity and less anti-factor IIa activity as compared to UFH. They act mainly by preventing thrombin generation and have lesser effect on a PTT as compared to UFH. Assays measuring anti-factor Xa activity are becoming available but are not yet in widespread use. Enoxaparin is the most popular of all LMWH that has been shown to be efficacious in patients with NSTE-ACS, as in acute pulmonary embolism and deep venous thrombosis. In the ESSENCE and TIMI 11B trials, enoxaparin has been demonstrated to have an advantage over intravenous heparin in reducing cardiovascular events in patients with NSTE-ACS. In a metaanalysis of these two trials, the risk of death, recurrent ischemia, and MI is reduced by about 20% by the use of enoxaparin as compared to UFH. In the ESSENCE trial, the benefit persisted for over a year. Similarly, dalteparin fared better than intravenous heparin in the FRISC trial in patients with ACS. The benefit was more pronounced in patients with high-risk features like troponin elevation and those with higher TIMI risk scores.
In patients with ACS who have creatinine clearance greater than 30 mL/min, enoxaparin is used in the dosage of 1 mg/kg subcutaneously twice daily. There is no need to monitor the clotting parameters because the therapeutic effect is quite consistent and predictable. The anticoagulant effect with enoxaparin is consistent because of very little binding to plasma proteins, endothelial cells, and macrophages. With newer assays being made available, one may soon be able to monitor anti-factor Xa activity when using enoxaparin. Enoxaparin’s risk of thrombocytopenia is quite low. Major bleeding is also uncommon with enoxaparin, but the risk may be higher in the elderly and those with renal failure. In patients requiring CABG, the drug should be stopped 12 to 24 h prior to the operation. In patients undergoing cardiac catheterization and PCI, there is always a concern for bleeding because of concomitant use of UFH, Gp2b/3a receptor antagonists, and clopidogrel. The following rule of thumb can be used for heparin dosing in patients needing PCI: within 8 h of having received a dose of enoxaparin, no additional UFH is needed for PCI; between 8 and 12 h, use UFH at dose of 25 to 50 units/kg; and if greater than 12 h after receiving enoxaparin, use 50 to 70 units/kg of UFH. Despite its proven efficacy, only about 15% of patients in North America and 50% of patients in Europe receive LMWH for ACS.
Direct Thrombin Inhibitors
The direct thrombin inhibitor (DTI) agents available are hirudin, lepirudin (recombinant hirudin), argatroban, and bivalirudin. These agents are substantially more expensive than UFH and enoxaparin. They are powerful anticoagulants and their anticoagulation effect is consistent and predictable. DTIs do not depend on antithrombin III for their activity. They bind to thrombin (factor IIa) and thus inhibit coagulation process. Because thrombin is also a powerful platelet activator, DTIs also inhibit platelet activation. In a large metaanalysis, DTIs were shown to reduce rates of recurrent ischemia and infarctions as compared to heparin in patients with NSTE-ACS, but their use was associated with increased incidence of major bleeding requiring blood transfusions. DTIs are currently only recommended for those with HIT. However, the use of Bivalirudin in the setting of coronary intervention is increasing, ever since the REPLACE-2 trial showed significantly reduced procedure associated bleeding rates compared with heparin and Gp2b/3a receptor antagonists. This drug although expensive has become more popular with interventional cardiologists.
Fibrinolytic Therapy
There is no proven benefit of fibrinolytic therapy in NSTE-ACS. This is probably because a completely occlusive coronary thrombus is present in fewer than 50% of patients, and also because platelet-rich thrombi which predominate in coronary vessels of patients with NSTE-ACS are resistant to dissolution with fibrinolytic therapy. Fibrinolytic agents have not been demonstrated to be effective in reducing the risk of MI or death in NSTE-ACS and in fact may be deleterious. This is in stark contrast to STEMI-ACS, where the benefit of fibrinolytic therapy is proven. Therefore, fibrinolytics are contraindicated in NSTE-ACS.
Invasive Strategy of Coronary Angiography and Percutaneous Coronary Intervention
Several recent studies have demonstrated benefit with early invasive strategy in patients with NSTEACS as compared to conservative treatment strategy. In the early invasive strategy, patients undergo coronary angiography and revascularization within 12 to 48 h of presentation to the hospital with ACS. In the conservative strategy, patients underwent coronary angiography only for significant recurrent ischemia or ischemia demonstrated by stress testing. The early invasive strategy results in less short-, intermediate-, and long-term major cardiac event rates (death, MI, recurrent ischemia, and revascularization rates) and shorter lengths of stay in the hospital. This is particularly true in patients with high-risk characteristics like elevated serum cardiac biomarkers (like troponins), ongoing chest discomfort, and dynamic ST-T changes on ECG. In intermediate-risk patients, a conservative strategy may be as good as an early invasive strategy. In low-risk patients, a conservative strategy is preferred.
It has been shown that use of aggressive medical regimens including “upstream” use of Gp2b/3a receptor antagonist (tirofiban or eptifibatide) for 12 to 24 h before PCI reduces the risk of MI or death after PCI by at least 30% to 40%. The majority of patients with NSTE-ACS will be candidates for PCI after coronary angiography (70% to 80%). Compared to balloon angioplasty, coronary stenting appears to substantially reduce recurrent ischemia and infarction. Restenosis in 3 to 6 months is
a major limitation with bare-metal stents and occurs because of intimal hyperplasia reaction to the vessel wall injury. Since 2003, there has been a widespread use of DES in the United States, which reduces long-term restenosis and repeat revascularization rates by 50% to 70%. However, these patients have to remain on long-term clopidogrel and asprin therapy.
a major limitation with bare-metal stents and occurs because of intimal hyperplasia reaction to the vessel wall injury. Since 2003, there has been a widespread use of DES in the United States, which reduces long-term restenosis and repeat revascularization rates by 50% to 70%. However, these patients have to remain on long-term clopidogrel and asprin therapy.
Emergent cardiac catheterization and revascularization in NSTE-ACS is needed less commonly. The indications include pulmonary edema, hypotension, and malignant ischemic ventricular arrhythmias. Most of the other high-risk patients can be stabilized with medical management for 12 to 48 h before angiography and revascularization.
Coronary Bypass Graft Surgery Versus Stenting
The mortality risk with urgent CABG in NSTE-ACS patients is around 4% to 5%. The other complications of bypass surgery include stroke and cognitive abnormalities. This is mainly due to cross-clamping of the aorta and the use of cardiopulmonary bypass. This should be borne in mind especially while operating on elderly patients. The complications and recovery times have improved over the course of the two decades because of refinement in surgical techniques and postoperative care. The advent of left internal mammary artery grafting to the left anterior descending artery was a major advance in bypass surgery since the 1980s. The use of offpump bypass surgery may reduce the risk of stroke in elderly patients.
The usual length of stay in the hospital is 5 to 7 days, but it may take upto 2 to 3 months for the patients to recover back to their usual baseline.
Only 20% to 30% of NSTE-ACS patients need urgent CABG. The classical indications for CABG include (a) significant left main coronary stenosis, (b) multivessel coronary artery disease (CAD) with left ventricular ejection fraction (LVEF) less than 40%, (c) CAD with significant valvular disease (aortic stenosis and mitral insufficiency), (d) diabetes mellitus with multivessel CAD, (e) coronary anatomy unsuitable for PCI, and (f) failed PCI. It is preferable to stabilize these patients with medical management prior to CABG. Sometimes an intraaortic balloon pump (IABP) may be needed for prior stabilization in patients with hypotension, CHF, and LV dysfunction. However, with the ever-expanding horizons of interventional cardiology many of the patients who previously would have gone for bypass surgery are now receiving DES. The debate of which is better (bypass surgery or stenting) in patients with complex coronary disease (multivessel CAD, total occlusions, left main coronary artery disease, etc.) continues.
The recent SYNTAX trial has compared use of DES (paclitaxel-eluting) to CABG surgery in patients with over 1,800 patients with complex coronary artery disease who were randomized to either bypass surgery or multivessel stenting. The combined endpoint of death, repeat revascularization, stroke, and MI at 1 year favored bypass surgery. The differences were driven mainly by higher repeat revascularization rates in the stent arm of the trial. The risk of death or MI was no different in the two arms. The risk of stroke was more than three times higher in the surgical group. This trial although providing some clear insights has by no means put to rest the raging debate. The recommendation therefore is to individualize therapy after taking into considerations the following factors: (a) coronary anatomy; (b) LV function; (c) comorbid conditions; (d) age of the patient; and (e) patient’s wishes.
Intraaortic Balloon Pump
An IABP may prove useful for hemodynamic stabilization while awaiting PTCA or CABG, particularly for patients with LV dysfunction, CHF, hypotension, or acute mechanical defects (e.g., mitral regurgitation [MR] or ventricular septal defect [VSD]). Balloon inflation during diastole augments coronary perfusion and deflation during systole decreases LV afterload. Unless a rapidly correctable mechanical defect is present, the use of IABP does not improve outcomes.
Risk Factor Modification
For the patient who has been stabilized medically or following revascularization procedures, risk factor modification is essential in preventing recurrent ischemia, infarction, and sudden death from progression of CAD. Smoking cessation, control of diabetes mellitus and hypertension, correction of abnormal lipid patterns, and weight reduction are critical elements in risk factor modification. Most should remain on aspirin, β-blockers, statins, and angiotensin-converting enzyme inhibitors (ACEI). Establishing a regular program of exercise is pivotal in achieving these goals and improving exercise tolerance. Patients with good exercise capacity are known to have fewer cardiovascular events and seem to tolerate them better.
▪ ACUTE CORONARY SYNDROMES: ST ELEVATION MYOCARDIAL INFARCTION (ACS-STEMI)
Mechanisms
STEMI results from plaque rupture and formation of superimposed thrombus. The thrombus that causes complete occlusion of a major coronary artery is usually rich in fibrin and red blood cells (“red-clot”). This is in contrast to the thrombus seen with NSTEACS, which is characterized by formation of a platelet-rich thrombus (“white-clot”). A wave of myocardial necrosis spreads from the endocardium to the epicardium with complete coronary occlusion. The process of infarction is usually completed in 24 h, and it is called a “full-thickness” or completed infarction. Q waves are typically seen in the ECG with a completed or full-thickness infarction. If angiography is performed promptly, a fresh occlusive coronary thrombus may be demonstrated in most cases (approx. 90%). Nonthrombotic spasm of the coronary arteries in an area of atherosclerosis is responsible for a small fraction of AMIs. Rarely, coronary flow may be interrupted by embolism in patients with endocarditis, prosthetic valves, or rheumatic valvular disease. Only 5% to 10% of patients sustaining an MI have normal coronary arteries. (Although spontaneous thrombolysis of clot is suspected, the mechanism of infarction in these cases usually remains unknown.) Cocaine is responsible for an alarming number of MIs. Because cocaine enhances platelet aggregation, causes vasoconstriction, and increases heart rate through catecholamine-mediated mechanisms, it can produce infarction even in patients with normal coronary arteries.
Diagnosis
History
Classical Presentation The typical presentation is one characterized by the abrupt onset of left-sided or retrosternal chest, neck, and jaw discomfort, which has been described as burning, squeezing, or pressurelike sensation lasting for more 30 min. The discomfort may radiate to the arms, neck, back, or jaw. It must be emphasized that the pain description may be highly atypical (burning, stabbing, sharp) or may be localized only to the arm or neck. Autonomic symptoms (nausea, vomiting, sweating) are more common than in UA. Up to 20% of MIs are painless (more likely in diabetics and the elderly). Young age, paucity of classic risk factors, and atypical chest pain character are more common in patients with cocaine-induced infarction.
Atypical Presentation Patients may describe pain as being sharp or stabbing. The pain may be localized to the arm, shoulder, or neck. Symptoms may mimic gastroesophageal reflux, cholecystitis, or an acute abdomen. Acute onset of shortness of breath, heart failure, dizziness, syncope, and weakness have all been described as atypical manifestations of an AMI. Atypical presentations are seen in women, diabetics, elderly, and cocaine and other drug overdose states.
Silent MI Clinically silent infarcts are detected incidentally on an ECG, echocardiogram, or nuclear scan. Silent MI is usually seen in diabetics with autonomic dysfunction.
Physical Examination
Blood pressure and pulse rate usually are mildly increased. (Tachycardia is more common in anterior or lateral MI than in inferior or posterior MIs, in which bradycardia is more likely.) Fever may accompany uncomplicated MI but rarely exceeds 101°F or persists beyond 1 week. An S4 gallop is very common, whereas an S3 suggests congestive failure, especially if accompanied by pulmonary rales. A paradoxically split S2 indicates increased LV ejection time or may be from a left bundle branch block (LBBB). A systolic murmur should raise the suspicion of acute papillary muscle dysfunction, especially if the patient has presented late (typically, a few days after onset of symptoms). A pericardial friction rub commonly appears in the first 48 h after MI and may be easily confused with a murmur. Although also possible in a classic MI, findings of a hyperadrenergic state (mydriasis, agitation, hypertension, diaphoresis, and/or tachycardia) should raise suspicion of cocaine-induced infarction.
Electrocardiogram
ST Segment Deflection ST elevation greater than or equal to 1 mm in two or more contiguous leads is highly suggestive of STEMI. ST elevation has a high localizing value (Table 21-1). The typical ST elevation seen with STEMI has an outward convexity. The ST segment elevations usually return to baseline with myocardial reperfusion and can be used to monitor reperfusion
therapies. The differential diagnosis includes hyperkalemia, acute central nervous system (CNS) injury, acute myocarditis, acute pericarditis, left ventricular hypertrophy, apical cardiomyopathy, Wolff-Parkinson-White syndrome, early repolarization abnormalities, and LV aneurysm. Some of these may mimic an AMI and hence have been termed “pseudoinfarct” pattern.
Evolution of ECG Changes A series of repolarization changes are seen on ECG with complete coronary artery occlusion. The first transient abnormalities seen are the hyperacute T waves (tall, peaked, and symmetrical T waves). Hyperacute T waves are usually gone by the time of initial presentation for emergency care. This is followed by convex, upward ST elevation, which is a sign of transmural myocardial ischemic injury. The number of leads showing the abnormality has a bearing on the size of the infarction and prognosis. T wave inversions are seen with persistent transmural ischemia. By this time, the ST elevations have begun to subside. Q waves are a sign of completion of the infarction and may take hours to days to develop. Persistent ST elevation beyond 3 to 4 weeks is sign of an LV aneurysm.
Posterior MI This manifests as ST depression (≥2 mm) in leads V1 to V3. It is usually seen along with an inferior wall MI, but can present on its own as a true posterior infarction. This is seen with either left circumflex or distal RCA occlusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree