Key Clinical Questions
What are the available tools to diagnose and risk stratify patients with suspected acute coronary syndrome (ACS)?
What are the American College of Cardiology and American Heart Association (ACC/AHA) class I guideline recommendations for anti-ischemia and antithrombotic therapy for unstable angina (UA) and non-ST-elevation myocardial infarction (NSTEMI)? For STEMI? Which drugs reduce mortality?
What are the benefits of an invasive versus a conservative strategy for managing UA/NSTEMI?
Which patients should undergo early invasive therapy in the setting of ACS?
Introduction
The term acute coronary syndrome (ACS) refers to a group of clinical symptoms consistent with acute myocardial ischemia, whether from unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), or ST-segment elevation myocardial infarction (STEMI). The definition of STEMI is based on the electrocardiographic (ECG) criteria of ST-segment elevation, and this diagnosis accounts for 330,000 hospital admissions per year in the United Sstates. The definition of NSTEMI is based on the absence of ST-segment elevations on the ECG, but ST depression and T-wave inversions may be evident, and there is sufficient ischemic damage to cause biochemical evidence of myonecrosis. NSTEMI accounts for 570,000 hospital admissions per year. UA and NSTEMI have similar pathogenesis and clinical presentations, but UA is not associated with release of cardiac troponins and/or creatine kinase (CK-MB). UA accounts for 670,000 admissions per year. According to the American Heart Association (AHA), an estimated $177 billion has been spent on the treatment of coronary heart disease in 2010.
Although tremendous strides have been made in the management of cardiovascular disease, coronary heart disease currently accounts for almost 20% of deaths in the United States. The 30-day mortality associated with the diagnosis of ACS varies from 1.7% for patients with UA to 7.4% for patients with NSTEMI to 11.1% for those with STEMI.
Despite chest pain pathways to guide clinicians in risk stratification, approximately 5% of symptomatic patients with myocardial infarction are inappropriately discharged from the emergency department to the outpatient setting. In addition, nearly 50% of all myocardial infarctions (MIs) produce no symptoms.
This chapter will focus on patients diagnosed with ACS, encompassing STEMI, NSTEMI, and UA, and review the best medical evidence based on the latest pertinent clinical trials reporting diagnosis and management of ACS. Recommendations will be referenced according to the American College of Cardiology (ACC)/AHA guidelines for the management of patients with STEMI and UA/NSTEMI along with the 2007 focused update of these guidelines. This chapter will not review the rapid rule-out protocols for the more than 75% of 5 million patients who present to emergency departments in the United States annually and have no evidence of ACS. Noncardiac chest pain and chronic stable angina are covered elsewhere in this book (Specific treatments/interventions may be described in relation to ACC/AHA guideline recommendations as per the class scheme explained in Table 124-1).
| Class I | Class IIa | Class IIb | Class III |
|---|---|---|---|
| Benefit > > > Risk | Benefit > > Risk | Benefit ≥ Risk | Risk ≥ Benefit |
| Additional studies with focused objectives needed | Additional studies with broad objectives needed; additional registry data would be helpful | No additional studies needed | |
|
| Procedure/Treatment MAY BE CONSIDERED |
|
|
|
|
|
Pathophysiology
The key event in the vast majority of ACS presentations concerns the abrupt disruption of a preexisting fibrous plaque involving primarily the intima of large- and medium-sized coronary arteries. Coronary artery thrombosis may result from complete plaque rupture, superficial plaque erosion, or a calcified plaque nodule. The exact mechanisms whereby a previously stable plaque is rendered “unstable” are not fully understood, but a key role for inflammatory cells has been advanced in the development of thin-cap fibroatheroma located predominantly in the proximal and mid sections of the three major coronary arteries and, importantly, within areas of <50% arterial stenosis. Accurately predicting vulnerable plaques in the clinical setting is difficult because plaque composition rather than size affects both the likelihood of rupture and the exposure of prothrombotic material such as cholesterol, collagen, and fibrin to platelets, and coagulation cascade elements to the endothelium.
Transmural ischemia from occlusion of a coronary thrombus causes STEMI; whereas nonoccluding thrombus leads to UA or NSTEMI (Figure 124-1).
Decreased blood delivery to the downstream myocardium, often due to a partially occlusive thrombus overlying a disrupted atherosclerotic plaque, from microembolization of thrombus and plaque debris with resulting blockage of the distal microcirculation
Fixed severe narrowing caused by progressive atherosclerosis or in-stent restenosis resulting in sufficient collateral blood flow around the stenosis to prevent transmural infarction when a fully occlusive thrombosis occurs
Dynamic obstruction caused by intense vasospasm, often referred to as Printzmetal angina, cocaine-induced vasoconstriction, or other causes of vasoconstriction
Inflammatory or infectious initiators of arterial narrowing, plaque rupture, and/or thrombogenesis
Increased myocardial oxygen demand (fever, tachycardia, hyperthyroidism, and other hyperadrenergic states, elevations of left ventricular afterload from hypertension, severe aortic stenosis, hypertrophic cardiomyopathy) and reduced myocardial oxygen delivery (anemia, hypoxemia, hypotension) despite normal coronary arteries
Diagnosis
The 2007 ACC/AHA guidelines for managing UA and NSTEMI recommend that practitioners use the patient’s history, physical examination, ECG interpretation, and results of cardiac biomarkers to determine the likelihood that the patient’s presentation is ACS from obstructive coronary artery disease (CAD). The diagnosis for STEMI is made on the basis of ST-segment elevation in the appropriate clinical setting.
Classic ACS-associated symptoms include chest, upper limb extremity, and jaw or epigastric pain or discomfort at rest or associated with exertion or emotional stress. In general, diffuse, nonpositional, poorly localized chest pain or discomfort is present in three-quarters of patients presenting with acute MI. Patients may also describe upper abdominal pain or associated symptoms such as shortness of breath, diaphoresis, nausea, and presyncope or syncope. Symptoms persisting for more than 20 minutes suggest infarction rather than UA. Diabetic patients or other patients with autonomic neuropathy, women and elderly patients, and postoperative patients may not exhibit the classic symptoms of ACS. They may present with hypotension that is unexplained, pulmonary edema, delirium, or profound weakness. Vigorous physical exercise such as shovelling snow, intense emotion such as arguing with a family member, and medical or surgical stress may precipitate STEMI in as many as 50% of cases. Circadian variations may be a factor in clusters of cases within a few hours of awakening.
Initial evaluation
The differential diagnosis of ACS includes acute pulmonary embolism, aortic dissection, pericardial tamponade, tension pneumothorax, esophageal rupture, and other potentially life-threatening disorders. Once the diagnosis of ACS has been made, the 2007 ACC/AHA guidelines for managing UA and NSTEMI recommend that practitioners determine
|
The Braunwald classification system for UA hinges on the assessment of severity of the patient’s anginal symptoms (I. new-onset of severe angina or increasing angina not occurring at rest; II. angina at rest in the past month but not in the preceding 48 hours, III. rest angina within the past 48 hours), the clinical setting (A. unstable angina secondary to a non-cardiac condition that increases demand, B. primary unstable angina, and C. postinfarction angina within 14 days of acute MI), and presence or absence of accompanying transient ECG changes. Table 124-2 lists the Canadian Classification.
|
Although traditional cardiac risk factors are weak predictors of the likelihood of acute ischemia, their presence influences outcomes in patients diagnosed with ACS. For example, diabetic patients with UA/NSTEMI have a 50% greater risk of adverse cardiac outcomes than nondiabetic patients. Well-documented risk factors for obstructive CAD include age > 65, hypertension, hypercholesterolemia, diabetes, and tobacco exposure, and family history of CAD especially in a sibling. The examiner should also determine risk factors for other causes of life-threatening chest pain that would alter any management strategy using antithrombotic and antiplatelet agents. (See Chapter 77 for a more detailed discussion of chest pain.) Acute aortic dissection, pulmonary embolism, esophageal rupture, tension pneumothorax and cardiac tamponade may mimic the signs and symptoms of acute MI (Table 124-3).
| Feature | High Likelihood | Intermediate Likelihood | Low Likelihood |
|---|---|---|---|
| Presence of any of characteristics below | No high-likelihood factors and presence of any of characteristics below | No high or intermediate features but may have characteristics below | |
| History | Chest or left arm pain or discomfort as main symptom reproducing prior angina | CP or left arm pain as chief complaint | Probable ischemic symptoms |
| Age > 70 years | Recent cocaine use | ||
| Male | |||
| History of CAD, MI | Diabetes | ||
| Exam | Transient MR, hypotension, pulmonary edema, or rales | Extracardiac vascular disease | Chest discomfort reproduced by palpation |
| ECG | New transient ST-segment deviation (> 1 mm) or TWI in multiple precordial leads | Fixed Q waves | T-wave flattening or inversion < 1 mm in leads with dominant R waves |
| ST depression 0.5–1 mm or TWI > 1 mm | Normal ECG | ||
| Cardiac Biomarkers | Elevated cTnI, TnT or CK-MB | Normal | Normal |
Although most patients with chest pain will have an unrevealing physical examination, the examiner should first look for signs of cardiac compromise that may signify a large infarction and poor prognosis. Anterior MI may be associated with hypotension and tachycardia in approximately 25% of cases, whereas inferior MI may be associated with bradycardia and hypotension in up to 50% of cases. Low cardiac output may be suggested by the presence of low blood pressure, significantly reduced mean arterial pressure compared to the patient’s baseline, and sinus tachycardia accompanied by diaphoresis, pale cool skin, confusion, and reduced urine output. Signs of elevated filling pressures include a palpable or audible S3, audible S4, elevated jugular venous distention, or rales. A midsystolic or late-systolic apical murmur may result from mitral valve dysfunction. Right ventricular infarction may have signs of hypotension (eg, elevated jugular venous distention) without signs of left ventricular failure. A transmural MI may produce a transient pericardial friction rub. The examiner should also look for signs of precipitating factors (eg, severe hypertension, hyperthyroidism, hemorrhage or other causes of severe anemia, and hypoxia) and for signs of vascular disease.
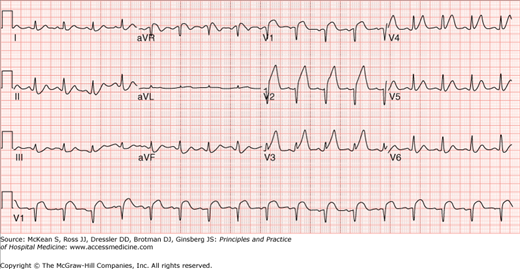
ECG interpretation facilitates making the diagnosis of ACS and stratifying risk. Despite limitations of the ECG in signifying ischemia of the posterior, lateral, and apical walls of the left ventricle, the admission ECG strongly predicts both early and long-term prognosis. Although a normal ECG on admission may be associated with as high as a 10% incidence of NSTEMI, and an acute MI resulting from left circumflex artery occlusion may be missed in at least 4% of cases, patients with no ECG findings have a lower risk of complications than those with new ECG abnormalities. ST deviation of only 0.5 mm (0.05 mV) increases the 30-day and 1-year risk of mortality or MI twofold.
The ACC/AHA guidelines recommend obtaining an ECG within 5 minutes of acute chest pain presentation so that it can be interpreted by an experienced physician within 10 minutes of arrival.
The patient should be placed on a cardiac monitor in immediate reach of resuscitation equipment including a cardiac defibrillator. Patients should have a bedside chest plain film that screens for alternative diagnoses and for pulmonary congestion (associated with an adverse prognosis). A bedside two-dimensional echocardiograph will detect abnormalities of wall motion in all cases of STEMI and, thus, may be indicated when there is uncertainty about the diagnosis (eg, pericardial effusion, RV infarction, ventricular aneurysm, or LV thrombus), the need for emergent reperfusion therapy, or prognosis (eg, reduced left ventricular function).
If the patient is experiencing a STEMI, a decision with regard to reperfusion strategy should be made as quickly as possible (within 10 minutes of arrival to the health care facility). ST-segment elevation of 1 mm (0.1 mV) or more in two or more contiguous leads or new left bundle-branch block (LBBB) is highly indicative of acute MI in 90% of patients. Sgarbossa’s criteria refers to the ECG criteria for identifying acute MI in patients with LBBB or ventricular pacing: (1) ST-segment elevation of ≥ 5 mm in the presence of a negative QR complex, (2) ST-segment elevation of ≥ 1 mm in the presence of a positive QRS complex, and (3) ST-segment depression of ≥ 1 mm in lead V1, V2, or V3. Additional ECGs encompassing right ventricular and posterior leads should be performed if inferior or posterior MI is suspected. ST-segment depression in two contiguous anterior precordial leads and/or lone ST-segment elevation in the posterior chest leads signify a true acute posterior MI. Right ventricular infarction accompanies inferior-posterior wall MI in 30% to 50% of patients. The diagnosis is made on the basis of ST-segment deviation of ≥ 1 mm (0.1 mV) in the right precordial lead V4R. Recognition is important due to the need for volume expansion, the possibility of advanced atrioventricular nodal block, and worse prognosis relative to patients suffering from interior-posterior MI without RV infarction. ECG interpretation is needed for identification of infarct territory in acute myocardial infarction (AMI) and to differentiate common nonischemic causes of ST-segment elevation including early repolarization, acute pericarditis, pulmonary embolism, left ventricular hypertrophy, Brugada syndrome, and others. (See Chapter 102 for a detailed discussion of electrocardiographic interpretation.)
Patients with ST-segment depression, transient ST-segment elevation, or deep symmetrical T-wave inversion (> 2 mV) are initially diagnosed with ACS until cardiac biomarkers distinguish between UA and NSTEMI. Lesser degrees of ST-segment deviation (< 0.5 mV) and/or T-wave changes are less specific, but the more leads that have these “nonspecific” changes, the greater is the likelihood of an acute NSTEMI rather than UA.
The ACC/AHA guidelines recommend serial ECG tracings or continuous ST-segment monitoring for patients with UA and NSTEMI.
Measurement of the cardiac troponins (T or I), may provide confirmatory evidence of myocardial necrosis within two to three hours in 80% of patients with acute MI. Current guidelines advocate repeated cardiac troponin (cTn) measurements at 6 and 12 hours post symptom onset to exclude MI. The biomarker of choice, cTn elevation, almost always reflects myocardial necrosis and has proved to be a powerful predictor of mortality in those with ACS. False positives may arise due to cross-reacting antibodies or as a consequence of fibrin interference. Between 15% and 53% of patients with end-sage renal disease (ESRD) may have positive cTnT measurements in the absence of myocardial necrosis compared with 10% for cTnI. However, all elevations of cTn, even in those patients without demonstrable myocardial necrosis, such as those with falsely elevated cTnT in the setting of severe renal dysfunction, are associated with increased morbidity.
The admission ECG
Prognosis
When the initial ECG is not diagnostic
|
CK-MB remains part of a multi-marker test to diagnose early MI and continues to be very useful in the diagnosis of re-infarction or infarct extension or peri-procedural MI due to its shorter half-life. Commonly, CK-MB levels are relied upon to estimate infarct size despite evidence to suggest that cTn (at 72–96 hours) may reflect the extent of myocardial necrosis with greater accuracy.
Elevated biomarkers
Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002 Apr 16;105(15):1760–1763. |
Triage of Patients with ACS
The 2007 ACC/AHA guidelines for ACS recommend early stratification for risk of short-term death or nonfatal MI to determine the site of care, specific therapy, approach (early invasive versus conservative strategy), and prognosis. Treatment of possible, probable, or definite ACS should occur in a chest pain unit (CPU), medical telemetry unit, or coronary care unit (CCU) depending on the level of suspicion or confirmation of ACS. Low-risk patients can be evaluated in a CPU or medical telemetry unit. Patients with evidence of STEMI or other significant ECG changes, as well as those with any hemodynamic instability or new arrhythmia, should be evaluated in the CCU setting.