Fig. 15.1
A patient who has been diagnosed with acute acalculous cholecystitis. The gallbladder has a characteristic thickened wall with dependent sludge in the gallbladder. The patient was successfully treated with percutaneous drainage
Laparoscopy
The primary utility of laparoscopy for AAC is when the diagnosis is in doubt or if percutaneous cholecystostomy has failed to correct the patient’s illness [82–85]. Bedside laparoscopy has been used with some success for the diagnosis and therapy of AAC but initial enthusiasm has waned because bringing the equipment to the ICU bedside is cumbersome. Nowadays due to advances in critical care anesthesia, most patients will tolerate the transport to the operating room as well as the physiologic effects of the anesthetic. Importantly, for severely inflamed gallbladders where complete laparoscopic cholecystectomy is not possible in an expedient manner, a laparoscopic damage control procedure may be performed to treat the patient’s pathology while minimizing the physiologic insult.
Therapy
In the past, the treatment for AAC was cholecystectomy [2], due to the ostensible need to inspect the gallbladder and perform a resection if gangrene or perforation was present. Other pathology that could mimic acute cholecystitis (e.g., perforated ulcer, cholangitis, pancreatitis) could also be identified at this time during open or laparoscopic operation if the diagnosis of AAC were incorrect. However, percutaneous cholecystostomy is now established as a lifesaving, minimally invasive alternative [86, 87]. Cholecystostomy by either technique will not decompress the common bile duct if cystic duct obstruction is present. Therefore the common duct must be decompressed in addition by some manner if cholangitis is suspected. Patency of the cystic duct can be determined immediately by tube cholangiography after cholecystostomy but this is usually not necessary. If gallstones are present an elective cholecystectomy is usually recommended in a delayed fashion whereas interval cholecystectomy is not generally indicated after AAC [87] and the cholecystostomy tube can be removed after tube cholangiography confirms that gallstones are absent.
Percutaneous cholecystostomy [88–90] controls the AAC in 85–90 % of patients. The gallbladder is usually intubated under sonographic (occasionally laparoscopic) control via an anterior or anterolateral transhepatic approach (through the right hepatic lobe) in order to minimize leakage of bile, but transperitoneal puncture has also been described. Rapid improvement should be expected when percutaneous cholecystostomy is successful. If rapid improvement does not ensue, suspicion should arise that the tube may be malpositioned and not draining properly, or the diagnosis of AAC may be incorrect. Perforated ulcer, pancreatic abscess, pneumonia, and pericarditis have been discovered in the aftermath of percutaneous cholecystostomy when patients failed to improve. Rarely, in genuine AAC, the patient will fail to improve due to gangrenous cholecystitis and an open procedure may be required [91, 92].
Reported major complications occur after 8–10 % of procedures, including dislodgment of the catheter, acute respiratory distress syndrome (ARDS), bile peritonitis, hemorrhage, cardiac arrhythmia, and hypotension due to procedure-related bacteremia [90]. The 30-day mortality of percutaneous and open cholecystostomy is similar, and influenced heavily by the underlying severity of illness.
Empiric percutaneous cholecystostomy has been advocated for patients who have sepsis absent a demonstrable source. In one report of 24 patients receiving vasopressor therapy for septic shock, 14 patients (58 %) improved as a result of cholecystostomy [89]. Pneumonia was diagnosed subsequently in three of the ten nonresponders, but an infection was never found in the other seven patients. Such an approach is not recommended routinely, but the importance of considering AAC in the differential diagnosis of occult sepsis is underscored.
Antibiotic therapy does not substitute for drainage of AAC, but is an important adjunct. The most common bacteria isolated from bile in acute cholecystitis are E. coli, Klebsiella spp., and Enterococcus faecalis, thus antibiotic therapy should be directed against these organisms. However, critical illness and prior antibiotic therapy alter host flora, and resistant or opportunistic pathogens may be encountered. Pseudomonas, staphylococci (including methicillin-resistant strains), Enterobacter and related species, anaerobic organisms (e.g., Clostridium spp., Bacteroides spp.), or fungi may be recovered. Anaerobes are particularly likely to be isolated from bile of patients with diabetes mellitus, in those older than 70 years of age, and from patients whose biliary tracts have been instrumented previously.
Complications
The prevalence of gallbladder gangrene in AAC exceeds 50 %, and leads to additional morbidity, including gallbladder perforation. One variant, emphysematous cholecystitis (Fig. 15.2), is particularly associated with gangrene and perforation. Emphysematous cholecystitis is rare, but shares many traits with AAC, as 28 % of patients with emphysematous cholecystitis have acalculous disease. More than 70 % of cases of emphysematous cholecystitis occur in men, and 20 % of patients have diabetes mellitus. Crepitus to palpation of the right upper abdomen or radiographic identification of gas in patients with acute cholecystitis warrants consideration for immediate cholecystectomy in view of the fulminant nature of untreated emphysematous cholecystitis. In this scenario, percutaneous cholecystostomy does not frequently achieve source control reliably enough and should only be used as temporizing measure in select circumstances. Importantly, if the patient does not improve, urgent cholecystectomy is needed.
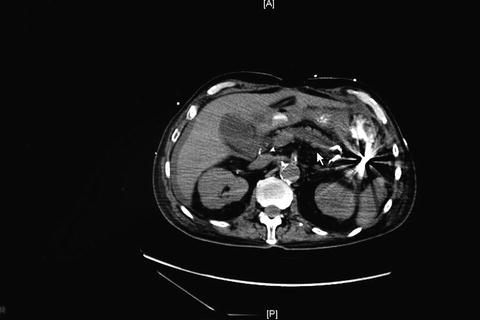

Fig. 15.2
A patient with emphysematous changes in acute acalculous cholecystitis. The gallbladder has a rim of air in the wall of the gallbladder adjacent to the liver bed
Clostridium spp., rather than aerobic gram-negative bacilli, are isolated most commonly in emphysematous cholecystitis (45 % of cases, with C. welchii predominating). Escherichia coli is recovered from approximately one-third of affected patients. Antimicrobial therapy specific for Clostridium (such as penicillin G) may be added to agents directed against the typical bacteria flora of acute cholecystitis.
Perforation of the gallbladder occurs in 10 % or more of cases of AAC [8], either localized into the subhepatic space or free perforation with generalized peritonitis. Perforation into the liver or biliary tract has been reported rarely in AAC [93, 94], as is perforation into the retroperitoneum with iliopsoas abscess [95]. Unusual causes of death from gallbladder perforation in AAC include hemorrhage from the liver [96, 102] and pulmonary bile embolism [97]. Serious complications of gallbladder gangrene without perforation include acute pancreatitis [98], colon perforation [99], and obstruction of the common hepatic duct [100]. Empyema of the gallbladder may also complicate AAC [101].
Conclusion
AAC should be suspected in every critically ill or injured patient with sepsis in whom the source of infection cannot be found immediately. Suspicion should be especially high if the patient has undergone recent major surgery, has had a period of hypoperfusion or becomes jaundiced. The preferred diagnostic modality is ultrasound, which is inexpensive, noninvasive, and can be brought to the bedside of the critically ill or unstable patient. Once diagnosed, the treatment of choice is percutaneous cholecystostomy. If the response to drainage is not prompt and favorable, an alternative diagnosis must be considered or abdominal exploration may be required. If percutaneous drainage is successful and the patient truly has no gallstones, then no further treatment may be necessary and the catheter may be removed after the patient has improved from the critical illness.
References
1.
Gallbladder Survey Committee. Ohio Chapter, American College of Surgeons. 28,621 cholecystectomies in Ohio. Am J Surg. 1970;119:714–7.
2.
Glenn F, Becker CG. Acute acalculous cholecystitis: an increasing entity. Ann Surg. 1982;195:131–6.PubMedCentralPubMed
3.
Treinen C, Lomelin D, Krause C, Goede M, Oleynikov D. Acute acalculous cholecystitis in the critically ill: risk factors and surgical strategies. Langenbecks Arch Surg. 2014. doi:10.1007/s00423-014-1267-6.
4.
Kalliafas S, Ziegler DW, Flancbaum L, Choban S. Acute acalculous cholecystitis: incidence, risk factors, diagnosis, and outcome. Am Surg. 1998;64:471–5.PubMed
6.
Barie PS. Acalculous and postoperative cholecystitis. In: Barie PS, Shires GT, editors. Surgical intensive care. Boston: Little, Brown; 1993. p. 837–57.
7.
Ouriel K, Green RM, Ricotta JJ, et al. Acute acalculous cholecystitis complicating abdominal aortic aneurysm resection. J Vasc Surg. 1984;1:646–8.PubMed
8.
Hagino RT, Valentine RJ, Clagett GP. Acalculous cholecystitis after aortic reconstruction. J Am Coll Surg. 1997;184:245–8.PubMed
9.
Cadot H, Addis MD, Faries PL, et al. Abdominal aortic aneurysmorrhaphy and cholelithiasis in the era of endovascular surgery. Am Surg. 2002;68:839–43.PubMed
10.
Leitman IM, Paull DE, Barie PS, et al. Intraabdominal complications of cardiopulmonary bypass surgery. Surg Gynecol Obstet. 1987;165:251–4.PubMed
11.
Gately JF, Thomas EJ. Acute cholecystitis occurring as a complication of other diseases. Arch Surg. 1983;118:1137–41.PubMed
12.
Fabian TC, Hickerson WL, Mangiante EC. Post-traumatic and postoperative acute cholecystitis. Am Surg. 1986;52:188–92.PubMed
13.
Gu MG, Kim TN, Song J, Nam YJ, Lee JY, Park JS. Risk factors and therapeutic outcomes of acute acalculous cholecystitis. Digestion. 2014;90(2):75–80.PubMed
14.
McDermott MW, Scudamore CH, Boileau LO, et al. Acalculous cholecystitis: its role as a complication of major burn injury. Can J Surg. 1985;28:529–33.PubMed
15.
Sanches BF, Martins T, Santos MJ, Azeredo P. Acute acalculous cholecystitis in a patient with juvenile dermatomyositis. BMJ Case Rep. 2014;19:2014.
16.
Newcombe JP, Gray PE, Palasanthiran P, Snelling TL. Q Fever with transient antiphospholipid antibodies associated with cholecystitis and splenic infarction. Pediatr Infect Dis J. 2013;32(4):415–6.PubMed
17.
Moolenaar W, Lamers CB. Cholesterol crystal embolization to liver, gallbladder, and pancreas. Dig Dis Sci. 1996;41:1819–22.PubMed
18.
Ryu JK, Ryu KH, Kim KH. Clinical features of acute acalculous cholecystitis. J Clin Gastroenterol. 2003;36:166–9.PubMed
19.
Smith JP, Bodai BI. Empyema of the gallbladder-potential consequence of medical intensive care. Crit Care Med. 1982;10:451–2.PubMed
20.
Ini K, Inada H, Satoh M, Tsunoda T. Hemorrhagic acalculous cholecystitis associated with hemodialysis. Surgery. 2002;132:903.
21.
Chung-Park M, Kim B, Marmyola G, et al. Acalculous lymphoeosinophilic cholecystitis associated with interleukin-2 and lymphokine-activated killer cell therapy. Arch Pathol Lab Med. 1990;114:1073–5.PubMed
22.
Lillemoe KD, Pitt HA, Kaufman SL, et al. Acute cholecystitis occurring as a complication of percutaneous transhepatic drainage. Surg Gynecol Obstet. 1989;168:348–56.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








