1. Liver tumors are rare in children and generally present as a painless mass.
2. Hepatoblastoma is the most common liver tumor in children.
3. Regional analgesia may be contraindicated in patients with liver tumors due to coagulopathy or tumor extension into the spinal canal.
4. Pheochromocytoma and paraganglioma may be functional and secrete catecholamines.
DISORDER: Hepatic tumors
BACKGROUND
Liver tumors are rare in infants and children. They typically present as a painless palpable or visible abdominal mass in the infant or older child, but advanced disease can present with anorexia, vomiting, weight loss, or abdominal pain. The most common liver tumor of childhood is hepatoblastoma (HB).
EMBRYOLOGY AND PATHOLOGY
• Hepatoblastoma: Represents almost 50% of hepatic tumors in children and 1% of all pediatric malignancies, frequently occurring before 3 years of age. It usually presents as an asymptomatic mass. As an embryonal tumor, it produces α-fetoprotein (AFP) that can be used to monitor treatment. HB may be associated with Beckwith-Wiedemann syndrome or other embryonal tumors such as Wilms tumor as well as familial adenomatoid polyposis. It has an increased incidence in low birth weight and very low birth weight infants (<1,000 g). Twenty percent of patients have distant metastasis at the time of diagnosis, with the most common sites being lung, brain, and bone.
• Hepatocellular carcinoma (HCC): Represents almost 25% of hepatic tumors in children. It may occur in association with cirrhosis from biliary atresia, Fanconi’s syndrome, or hepatitis B or various metabolic deficiencies such as tyrosinemia. Liver function tests can be abnormal, in contrast to HB. Children usually present with a dull aching pain and hepatomegaly. It is less readily resectable, and less responsive to chemotherapy than HB.
• Vascular tumors (e.g., hemangioma): Patients with large lesions and congestive heart failure may require embolization, medical, or surgical intervention for tumor reduction.
• Rare hepatic tumors include other malignant (sarcoma, cholangiocarcinoma) or benign (focal nodular hyperplasia [FNH], adenoma, hamartoma, cyst) tumors.
• Hepatic metastases in children may be from neuroblastoma, renal, or gastrointestinal (GI) tumors. Stage IV-S neuroblastoma may be associated with extensive liver disease but typically responds to treatment.
• Infiltration can occur from adjacent malignancies of the kidney or adrenal gland.
Staging: Historically, HB/HCC was staged at the time of surgery based on completeness of resection as follows:
• Stage I: Confined to liver, completely resected at initial surgery.
• Stage II: Confined to liver, microscopically positive margins.
• Stage III: Gross residual disease: unresectable or partially resectable, positive lymph nodes, tumor rupture from capsule.
• Stage IV: Distant metastases (lungs, brain, bone, bone marrow).
Since 1990, the International Society of Pediatric Oncology (SIOP) developed a presurgical staging system known as PRETEXT staging where the liver is divided into anterior, posterior, lateral, and medial segments and staging describes the number of segments involved:
PRETEXT I: One segment
PRETEXT II: Two segments
PRETEXT III: Three segments
PRETEXT IV: Four segments
Staging can be further described by P (portal vein involvement), V (hepatic vein involvement), E (extra-hepatic extension), or M (distant metastasis).
TREATMENT AND SURGERY
Complete surgical resection is the only cure for HB and HCC and remains the mainstay of treatment. For HB, chemotherapy is a useful adjuvant and neo-adjuvant therapy for shrinking the tumor and making it less vascular. Common agents used are doxorubicin, vincristine, 5-FU, cisplatin, and cyclophosphamide. HCC is resistant to systemic chemotherapy. The workup for liver tumors includes imaging studies, laboratory studies, and biopsy. Depending on the diagnosis and staging, chemotherapy (pre- and/or postresection), resection, resection of isolated metastases, less commonly radiation, or liver transplantation (for isolated unresectable liver tumors) may be required.
PROCEDURES REQUIRING ANESTHESIA
Depending on the patient’s age, degree of cooperation, and the type of procedure, pediatric patients with liver tumors may need sedation or anesthesia for the following:
• Imaging studies (ultrasonography, computed tomography [CT], magnetic resonance imaging [MRI], angiography, and nuclear scan)
• Biopsies
• Embolization
• Ethanol injection
• Central venous line (CVL) placement
• Staging laparotomy
• Tumor resection
• Radiation therapy
• Liver transplantation
ANESTHETIC ISSUES
CLINICAL PEARL Patients undergoing surgery for resection of hepatic tumors are at a high risk for significant cardiovascular compromise, large blood loss, temperature instability, and fluid and electrolyte perturbations.
READINESS FOR SURGERY AND ANESTHESIA
Because it is likely that an anesthetic may be necessary as early as the first diagnostic imaging tests, clinical evaluation and judgment is most important when patients present unprepared, untreated, and without a definite diagnosis. Patients scheduled for laparotomy, resection, central line placement for chemotherapy, or liver transplantation are typically extensively evaluated, allowing better risk analysis and planning by the anesthesiologist.
ANESTHESIA CONCERNS FOR PATIENTS WITH HEPATIC TUMORS
1. Hepatic effects: Impaired synthetic, metabolic, excretory function of the liver, altered pharmacokinetics of hepatically-eliminated drugs, hypoglycemia, coagulopathy.
2. Local effects: Mass effect on GI tract (gastric outlet obstruction), mass effect on the biliary tract, mass effect on and even infiltration into surrounding vascular structures including the inferior vena cava (IVC), right atrium (RA), celiac trunk, or renal vessels and other structures such as the diaphragm or spinal canal.
3. Systemic effects (anemia, thrombocytopenia, hepatic encephalopathy).
4. Treatment effects (chemotherapy).
5. Surgical aspects (need for circulatory arrest, cardiopulmonary, or venovenous bypass in patients with tumor thrombus extending to the IVC).
PREPARATION FOR ANESTHESIA
1. Laboratory studies: Complete blood count (CBC); coagulation profile; hepatorenal panel, glucose, and electrolytes.
2. Blood bank: Clot to the blood bank, type and screen packed red blood cells (PRBCs) for biopsies, resection, and transplant, other blood products as indicated by studies; alert blood bank to possibly large blood product requirement including PRBCs, platelets, fresh frozen plasma (FFP), cryoprecipitate, factor VIIa.
3. Imaging studies: To be reviewed and assessed for mass effect on/extension into surrounding structures, size, location, and vascularity of tumor. Echocardiogram for assessment of contractility and for tumor effects of the RA or IVC.
4. Review preoperative chemotherapy (may include cisplatin, vincristine, 5-fluorouracil [5-FU], doxorubicin) and evaluate for side effects.
5. Preoperative treatment: Consider vitamin K, transfusion as indicated.
6. NPO time: Standard, but awareness of possible gastric outlet obstruction.
ANESTHESIA FOR RESECTION OF HEPATIC TUMORS
Anesthetic Plan
1. General anesthesia with tracheal intubation.
2. With or without epidural catheter depending on the surgical plan and the child’s coagulation studies.
3. Access and monitoring appropriate for major laparotomy with possibly massive bleeding.
4. Postoperative intensive care unit (ICU).
Induction
Concerns
1. Gastric outlet obstruction with risk of regurgitation.
2. Reduced functional residual capacity.
3. Poor chest compliance due to elevated diaphragm.
Depending on concerns about gastric outlet obstruction, an intravenous or rapid-sequence induction may be indicated or an inhalation induction may be appropriate. Orotracheal intubation is adequate. Antibiotics are administered per surgeon’s order.
Lines, Monitoring, and Additional Instrumentation
Standard American Society of Anesthesiologists (ASA) monitoring and
1. Arterial line (upper extremity).
2. Central line.
3. Large-bore venous access (upper extremities/neck).
4. Nasogastric tube.
5. Bladder catheter.
6. Temperature probe.
7. Consider precordial Doppler.
8. Intraoperative laboratory: Arterial blood gases (ABGs), CBC, coagulation studies, glucose, and electrolytes.
CLINICAL PEARL Regional analgesic techniques, while offering many advantages for patients undergoing resection of liver tumors, are often contraindicated in the immediate perioperative period due to concerns about the adequacy of coagulation and/or hemodynamic instability.
Regional Anesthesia
1. In addition to standard contraindications, special attention needs to be directed to possible tumor extension into the spinal canal, coagulopathy, or thrombocytopenia from hepatic disease or chemotherapy. An epidural catheter with midthoracic tip position for intra- and postoperative analgesia may be considered.
2. Intraoperative use needs to be balanced against potential aggravation of intraoperative hypotension by epidural local anesthetics.
Intraoperative Anesthesia
Concerns
1. Bleeding (from large vessels and liver parenchyma, reduced risk with current surgical technology).
2. Impaired venous return (lifting liver and kinking the IVC/hepatic veins, direct compression of IVC by surgeon).
3. Air embolism (from opening hepatic veins/IVC).
4. Poor chest compliance (from retractors under diaphragm).
5. Coagulopathy (baseline, but also from intraoperative bleeding, liver manipulation, and hypothermia).
6. Acute hemodynamic changes (from clamping/unclamping of the portal triad).
7. Arrhythmia (from mechanical irritation of the heart, electrolyte imbalances).
8. Pharmacokinetic considerations: potentially hepatotoxic or hepatically-eliminated drugs should be used with caution.
Intraoperative Management
1. Balanced anesthetic (narcotic/epidural—low-dose inhalational agent, neuromuscular blockade as indicated).
2. Epidural management dependent on blood pressure (BP).
3. Close BP monitoring with feedback to surgeons (mechanical factors).
4. Ventilation to normocapnia, FIO2 dependent on oxygenation, avoid nitrous oxide.
5. FFP early (typically coagulation abnormalities develop even in the absence of large blood loss).
6. Transfusion as indicated, blood products in the room.
7. Temperature management: Standard, including forced air warmer and fluid/blood warmer.
8. Check blood glucose, consider glucose containing maintenance fluid.
9. Consider renal protective measures if IVC clamping is required.
10. Inotropes, vasopressors, and vasodilators if clamping/unclamping of major vessels occurs.
Postoperative Considerations
1. Extubation in the operating room (OR) if appropriate, consider postoperative ventilation after long surgeries or massive transfusion.
2. Postoperative disposition: ICU or monitored intermediate care bed if extubated.
3. Postoperative analgesia: Epidural, nurse-controlled analgesia (NCA) or patient-controlled analgesia (PCA), or p.r.n. narcotics, careful with nonsteroidal anti-inflammatory medications and acetaminophen.
4. Postoperative coagulopathy: May persist without blood loss, coagulation checks, and FFP replacement as indicated. Check coagulation profile before epidural catheter removal.
5. Pharmacokinetic considerations: Beware of hepatically-eliminated (may accumulate) or hepatotoxic (acetaminophen) drugs.
DISORDER: Pheochromocytoma and paraganglioma
Pheochromocytoma and paraganglioma (PHEO/PGL) are neuroendocrine tumors notable for unphysiologic catecholamine release. Patients present with unexplained hypertension, tachycardia, palpitations, headaches, flushing, and sweating. Tumors may occur sporadically or as part of a hereditary tumor syndrome such as multiple endocrine neoplasia (MEN IIa, b), neurofibromatosis, von Hippel-Lindau syndrome, or tuberous sclerosis. PHEO/PGL is uncommon in neonates and infants (in whom catecholamine-secreting tumors are more typically neuroblastomas), but can be seen in children, adolescents, and adults.
EMBRYOLOGY AND PATHOLOGY
Neural crest cells migrate between endo- and ectoderm to form the adrenal medulla and sympathetic ganglia (see Fig. 24.1). Their transformation into pheochromocytes results in histologically characterized chromaffinic tumors in the adrenal (pheochromocytoma) or at the extra-adrenal sites of sympathetic ganglia (paraganglioma) in the neck, the chest, and retroperitoneum. Tumor cells retain the ability to secrete catecholamines. Pheochromocytomas may be histologically benign or malignant (~10%), and they may be bilateral (in ~10%).
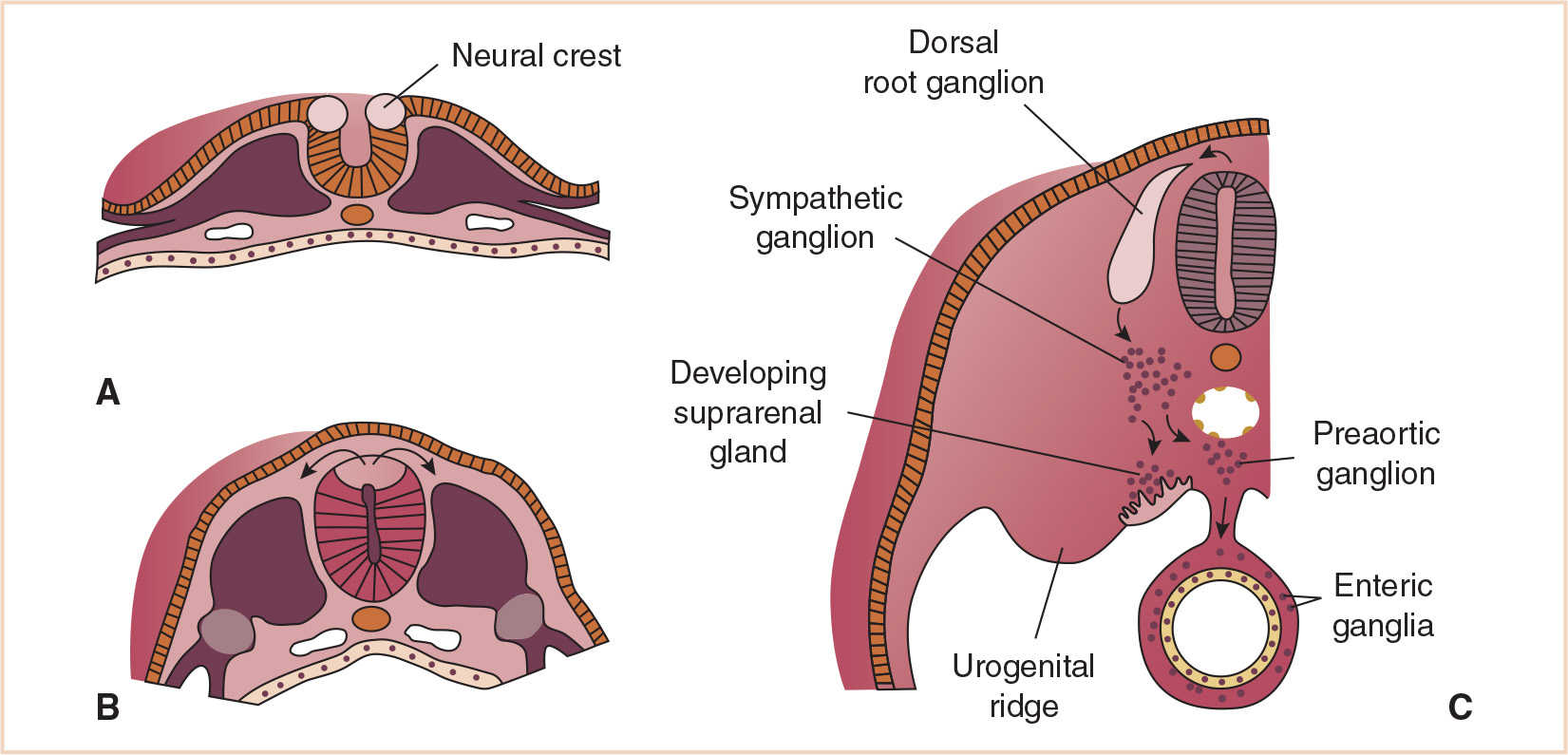
FIGURE 24.1 Development and migration of neural crest cells at 20 days (A), 22 days (B), and 28 days (C).
PHYSIOLOGIC CONSIDERATIONS
PHEO/PGLs can be functional or nonfunctional. Most PGLs of the head and neck are nonfunctional; however, abdominal PGLs are typically functional secretory tumors. Nonphysiologic secretion of catecholamines can be constant or episodic, resulting in increased levels of circulating norepinephrine and dopamine, less commonly epinephrine, with the physiologic effects of α-and β-adrenergic and dopaminergic stimulation by these catecholamines. The most obvious effects are increased vascular tone with hypertension and increased heart rate. If the predominant catecholamine secreted is norepinephrine secretion, the heart rate might be decreased. Circulating blood volume is reduced. Long-standing hypertension may result in ventricular hypertrophy and heart failure, or catecholamine cardiomyopathy. Preoperatively/preanesthetically, control of vasoconstriction and BP should be achieved. Alpha blockade is accomplished first, followed by blockade of the beta receptors. If beta blockade is the first intervention, the patient will have increased BP due to unopposed alpha activity.
Effective vasodilation will require concomitant hydration to restore circulating blood volume. Tumor removal will be associated with temporary catecholamine deficit, vasodilation, and hypotension, requiring additional fluid and vasopressors.
TREATMENT AND SURGERY
Resection of the tumor is typically curative. Malignant tumors may require additional chemotherapy. Medical treatment of hypertension due to catecholamine release is usually limited to preoperative BP control. Rarely, patients with symptoms have elevated catecholamine levels but a tumor cannot be identified. These patients are treated with long-term antihypertensive management with α-blockade.
DIAGNOSIS
Patients with PHEO/PGL typically present with symptoms from catecholamine release (hypertension, tachycardia, palpitations, headache, flushing, sweating). Rarely, PHEO/PGL can secrete other neuroendocrine hormones such as GNRH, CRH, ACTH, and PTHrP, and VIP. Symptoms can be spontaneous or provoked by physical activity, surgery, or anesthesia. Once suspected, PHEO/PGL diagnosis consists of biochemical confirmation and subsequent tumor location. Catecholamine (epinephrine, norepinephrine, dopamine) levels in blood and urine, catecholamine metabolites (vanillyl mandelic acid [VMA] and homovanillic acid [HVA]) in blood and urine, or free metanephrine and normetanephrine can be determined, either through random measurements or determination in a 24-hour urine collection. Urine metanephrine levels are the most sensitive assay because intratumor metabolism of catecholamines occurs independent of catecholamine release. Increasingly, patients are being diagnosed with genetic screening for hereditary tumor syndromes before the onset of symptoms. Once a biochemical diagnosis has been made, imaging studies (ultrasound, CT/MRI) identify the location of the tumor; iodine131-metaiodobenzylguanidine (131I-MIBG) nuclear scan investigates for tumor location and presence of metastases. Final diagnosis can be performed by histology following biopsy or resection. Presently there is no staging system for PHEO/PGL.
PROCEDURES REQUIRING ANESTHETIC INVOLVEMENT
Small children may require sedation and anesthesia care at an early stage during the diagnostic process when the final diagnosis is not yet established and treatment has not yet been initiated or optimized. Clinical evaluation and judgment is extremely important in these instances to avoid disastrous outcomes from anesthesia in a patient with an untreated pheochromocytoma. Preoperative biopsy is not indicated and can be dangerous, having caused hemorrhage, capsular disruption, hypertensive crises, myocardial infarction, stroke, and death.
• Diagnostic procedures: imaging studies
• Central line placement for chemotherapy
• Tumor resection
• Laparoscopic biopsy or tumor resection
CLINICAL PEARL Preoperative preparation for resection of a functional pheochromocytoma requires alpha blockade first, and then beta blockade while providing sufficient intravenous (IV) fluids and sodium intake to restore circulating blood volume.
ANESTHETIC ISSUES
READINESS FOR SURGERY AND ANESTHESIA
1. Hemodynamic control: If at all possible, hypertensive patients with clinical suspicion for catecholamine-secreting tumors should have their BP adequately controlled before an anesthetic. Classically, BP control occurs with α-adrenergic receptor blockers (phenoxybenzamine, prazosin, doxazosin, labetalol) over several days or weeks; however, other vasodilating drugs (urapidil, adenosine, PGE1, calcium channel blockers) have been used successfully. Once BP starts to normalize, β-adrenergic receptor blockade (metoprolol, labetalol) may be necessary for control of tachycardia. Beta blockade should not proceed without adequate α-adrenergic blockade for concerns of unopposed α-adrenergic stimulation. Short-acting α-blockers such as phentolamine and short-acting β-blockers such as esmolol are well suited for acute control of hemodynamics. Direct vasodilators such as sodium nitroprusside may also be used. Adequacy of blockade is often judged by nasal stuffiness and mild orthostasis in addition to BP normalization.
2. Restoration of circulating blood volume is assessed by absence of significant orthostatic hypotension, decrease in hematocrit, and increase in body weight. A few days prior to resection, increased dietary sodium intake or oral salt tablets can be initiated to increase intravascular volume retention. Patients are often admitted for, prior to surgery, IV hydration to prevent hypovolemia during preoperative fasting.
3. Available imaging studies are reviewed to evaluate location, size, mass effect, or invasiveness of tumor.
4. Available laboratory studies should include catecholamine levels, endocrine studies investigating presence of MEN, hepatorenal function tests, CBC, clotting studies, electrolytes, and blood glucose.
5. Cardiac evaluation (electrocardiogram [ECG], echocardiography) for adverse effects of long-standing hypertension, ventricular hypertrophy or strain, cardiac function, and cardiomyopathy. If cardiac changes are present, surgical resection may best be postponed until changes revert following medical management.
6. Funduscopy is performed to rule out retinal hemorrhage or papilledema from hypertension.
7. Blood bank: Clot to the blood bank for type, screen, and crossmatch of PRBCs.
ANESTHESIA CONCERNS
1. Adequacy of hemodynamic control: Severe hypertension with risk of stroke, acute cardiac failure, or bleeding can result from inadequate preanesthetic blockade and BP control. Invasive arterial BP monitoring, ideally instituted before anesthesia induction, is indicated. Central access for central administration of short-acting vasodilators is recommended.
2. Catecholamine release from the tumor can be
a. Spontaneous.
b. Drug-induced (histamine-releasing drugs).
c. Sympathetically mediated (light anesthesia).
d. Mechanical (tumor manipulation).
e. Due to laparoscopy (compression, CO2).
3. Local and mass effects of the tumor (pheochromocytomas are not necessarily large tumors, but relationship to surrounding structures needs to be determined).
4. Vasodilation and hypotension following tumor removal and temporary need for vasopressor support are common (neosynephrine, norepinephrine, dopamine, vasopressin infusion).
5. Choice of drugs: Almost all anesthetic drugs have been reported for the anesthetic management of patients with pheochromocytoma. Theoretic concerns exist with drugs that release catecholamines or histamines, drugs that are sympathomimetic or vagolytic, and with succinylcholine due to increased intra-abdominal pressure from fasciculations.
6. Hypercarbia-induced catecholamine release from hypoventilation and laparoscopy.
ANESTHESIA FOR RESECTION OF PHEOCHROMOCYTOMA/PARAGANGLIOMA
Anesthetic Plan
1. General anesthesia with tracheal intubation
2. Epidural catheter
3. Tight BP monitoring and management
4. Avoidance of drugs and maneuvers with risk of catecholamine release
5. Postoperative ICU
Induction
Concerns
1. Hypertension with induction in inadequately blocked patients, hypotension with excessively blocked patients.
2. Generous premedication is indicated to shield from stress.
3. Invasive arterial line placement before induction is recommended in order to closely follow hemodynamic changes with induction.
4. Vasodilators should be in-line peripherally and transferred to a central line as soon as available.
5. Topical local anesthetic to the larynx is useful in addition to generous doses of narcotics and induction agents to minimize the response to intubation. Orotracheal intubation is adequate.
Monitoring and Lines
Standard ASA monitoring and
1. Invasive arterial monitoring before induction.
2. CVL.
3. Nasogastric tube.
4. Bladder catheter.
5. Temperature probe.
6. Intraoperative laboratories (ABG, CBC, electrolyte, blood sugar as indicated).
Regional Anesthesia
Epidural anesthesia is suitable to suppress sympathetic stimulation thereby reducing the risk of sympathetically-mediated catecholamine release. Placement can occur in the awake, well-sedated patient, or in the asleep child after induction. The epidural catheter tip should be positioned midthoracic. In view of the presence of antihypertensive medication, the epidural catheter should be dosed carefully. Epidurally-mediated hypotension may aggravate intraoperative hypotension related to bleeding and to tumor removal.
Intraoperative Anesthesia
Concerns
1. Catecholamine release with hypertension with or without bradycardia renal vasoconstriction, tachycardia, and arrhythmias.
2. Intraoperative hypotension from bleeding, mechanically impaired venous return, and tumor removal.
3. Effects of laparoscopy.
Intraoperative Management
1. The key is tight hemodynamic monitoring and management.
2. Balanced anesthetic with narcotic/epidural and low-dose inhalational agent/total intravenous anesthesia (TIVA). Muscle relaxation as indicated with nonhistamine-releasing muscle relaxant such as rocuronium or vecuronium.
3. Intraoperative titration of short-acting intravenous vasodilators (sodium nitroprusside, phentolamine, calcium channel blockers, adenosine, prostacycline), short-acting β-adrenergic antagonists such as esmolol, or if longer-acting agents are needed, labetalol.
4. Magnesium sulfate as a bolus and maintenance infusion assists with BP control through vasodilation and its antiarrhythmic properties.
5. Fenoldopam has been used to counteract potentially detrimental renal–arterial vasoconstriction from catecholamine release.
6. With tumor resection, all vasodilators are discontinued, hypotension is managed by judicious fluid administration and intravenous vasopressor administration (neosynephrine, norepinephrine, vasopressin, dopamine, or epinephrine if needed).
7. With laparoscopy, insufflation pressure should be chosen as low as possible. Ventilation will aim for normocapnia.
CLINICAL PEARL It is important to be prepared for hypotension, resulting from deficit of catecholamines once the pheochromocytoma has been removed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








