Terms used to describe altered mental status (AMS) in critically ill patients include delirium, encephalopathy, acute confusional state, and acute brain failure.
 Terms such as “ICU psychosis,” “ICU syndrome,” or “ICU delirium” are misnomers and should be avoided.
Terms such as “ICU psychosis,” “ICU syndrome,” or “ICU delirium” are misnomers and should be avoided.
 Delirium is a neuropsychiatric syndrome characterized by:
Delirium is a neuropsychiatric syndrome characterized by:
 Disturbance of consciousness (i.e., reduced clarity of awareness of the environment) with reduced ability to focus, sustain or shift attention.
Disturbance of consciousness (i.e., reduced clarity of awareness of the environment) with reduced ability to focus, sustain or shift attention.
 A change in cognition or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia.
A change in cognition or the development of a perceptual disturbance that is not better accounted for by a preexisting, established, or evolving dementia.
 The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day.
The disturbance develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day.
 There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiological consequences of a general medical condition.
There is evidence from the history, physical examination, or laboratory findings that the disturbance is caused by the direct physiological consequences of a general medical condition.
Common Causes to Remember
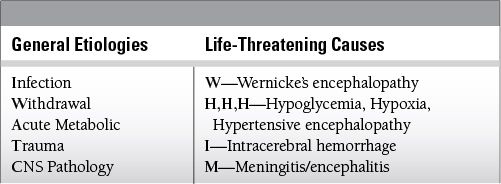
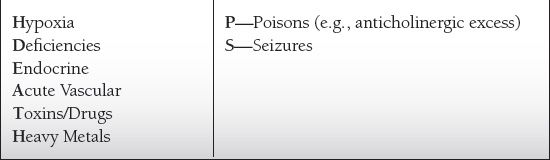
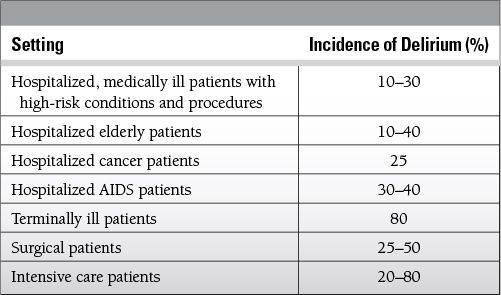
Epidemiology
Key Pathophysiology
 Several complementary theories have been proposed to explain the multifactorial processes of delirium.
Several complementary theories have been proposed to explain the multifactorial processes of delirium.
 Oxidative stress initiated by disturbances in brain structures most vulnerable to insult (selective vulnerability) with development of progression and dysfunction in other areas of the CNS (progressive vulnerability)
Oxidative stress initiated by disturbances in brain structures most vulnerable to insult (selective vulnerability) with development of progression and dysfunction in other areas of the CNS (progressive vulnerability)
 Inflammatory response with resulting proinflammatory-mediated cascade with disruption of the fenestrated endothelial zones leading to limbic disturbance
Inflammatory response with resulting proinflammatory-mediated cascade with disruption of the fenestrated endothelial zones leading to limbic disturbance
 Neurotransmitter dysfunction with changes in activity of cholinergic, dopaminergic, histaminergic, noradrenergic, and serotonergic neurons
Neurotransmitter dysfunction with changes in activity of cholinergic, dopaminergic, histaminergic, noradrenergic, and serotonergic neurons
 Disruption to the basal-ganglia-thalamo-cortical circuit nodal points leading to increased vulnerability to the development of delirium
Disruption to the basal-ganglia-thalamo-cortical circuit nodal points leading to increased vulnerability to the development of delirium
Differential Diagnosis
 Alcohol withdrawal—certain symptoms of alcohol withdrawal syndrome (fever, diaphoresis, tachycardia, and AMS) may overlap with that of SIRS and focus should be on evaluation of tremor and hypertension (elevated for active withdrawal and low to normal in SIRS), which generally will differentiate alcohol withdrawal from the latter.
Alcohol withdrawal—certain symptoms of alcohol withdrawal syndrome (fever, diaphoresis, tachycardia, and AMS) may overlap with that of SIRS and focus should be on evaluation of tremor and hypertension (elevated for active withdrawal and low to normal in SIRS), which generally will differentiate alcohol withdrawal from the latter.
 Dementia—dementia may be difficult to distinguish from delirium; however, collateral history of pre-existing cognitive dysfunction with slow onset differentiates it from delirium, which develops over hours to days.
Dementia—dementia may be difficult to distinguish from delirium; however, collateral history of pre-existing cognitive dysfunction with slow onset differentiates it from delirium, which develops over hours to days.
 Depression—hypoactive forms of delirium are often misdiagnosed as depression given symptom overlap of psychomotor slowing, cognitive changes, sleep disturbances, irritability, and in severe cases of depression, symptoms of psychosis and paranoia.
Depression—hypoactive forms of delirium are often misdiagnosed as depression given symptom overlap of psychomotor slowing, cognitive changes, sleep disturbances, irritability, and in severe cases of depression, symptoms of psychosis and paranoia.
 Attention and orientation are normal in depressed patients, however.
Attention and orientation are normal in depressed patients, however.
 Primary psychiatric disorders—symptoms of delirium may be caused by a primary psychotic disorder, or often in states of hyperactive delirium these symptoms are thought to be due to underlying anxiety and, therefore, are erroneously treated with benzodiazepines.
Primary psychiatric disorders—symptoms of delirium may be caused by a primary psychotic disorder, or often in states of hyperactive delirium these symptoms are thought to be due to underlying anxiety and, therefore, are erroneously treated with benzodiazepines.
 Visual hallucinations are markedly rare in primary psychotic disorders, whereas auditory hallucinations are uncommon in delirium.
Visual hallucinations are markedly rare in primary psychotic disorders, whereas auditory hallucinations are uncommon in delirium.
 Furthermore, consciousness, attention, and cognition are generally not as impaired (if at all) in primary psychoses.
Furthermore, consciousness, attention, and cognition are generally not as impaired (if at all) in primary psychoses.
Management and Treatment
 Definitive treatment involves quick and accurate identification of the underlying causes of delirium with the goal of correcting the underlying etiology(ies).
Definitive treatment involves quick and accurate identification of the underlying causes of delirium with the goal of correcting the underlying etiology(ies).
 Confusion Assessment Method—ICU or the Intensive Care Delirium Screening Checklist (ICSDSC) are both reasonable screening tools for the presence of delirium, and the ICDSC may also be used to track severity.
Confusion Assessment Method—ICU or the Intensive Care Delirium Screening Checklist (ICSDSC) are both reasonable screening tools for the presence of delirium, and the ICDSC may also be used to track severity.
 Management strategies
Management strategies
 Monitor vital signs, fluid intake and output, and oxygenation
Monitor vital signs, fluid intake and output, and oxygenation
 Treat dehydration, heart failure, and electrolyte disorders
Treat dehydration, heart failure, and electrolyte disorders
 Treat common infections: urinary, respiratory, soft tissue
Treat common infections: urinary, respiratory, soft tissue
 Treat severe anemia (transfusion), hypoxia, and hypotension
Treat severe anemia (transfusion), hypoxia, and hypotension
 Identify and manage sources of pain
Identify and manage sources of pain
 Maintain aspiration precautions
Maintain aspiration precautions
 Discontinue nonessential medications
Discontinue nonessential medications
 Assess and treat urinary retention and fecal impaction
Assess and treat urinary retention and fecal impaction
 Anticipate and prevent immobility and falls
Anticipate and prevent immobility and falls
 Avoid interruption of sleep if possible
Avoid interruption of sleep if possible
 Mobilize with assistance and physical therapy as early as clinically feasible
Mobilize with assistance and physical therapy as early as clinically feasible
 Pharmacologic strategies
Pharmacologic strategies
 Dopamine antagonists
Dopamine antagonists
 Haloperidol
Haloperidol
 Intravenous haloperidol is the first-line agent for the treatment of agitated delirious patients in the ICU setting due to its minimal effects on heart rate and BP. Dosing range is generally 2 to 20 mg IV.
Intravenous haloperidol is the first-line agent for the treatment of agitated delirious patients in the ICU setting due to its minimal effects on heart rate and BP. Dosing range is generally 2 to 20 mg IV.
 Dose should be started at a lower dose and doubled every 15 minutes until the patient is calm (maximum single bolus being 20 to 40 mg IV).
Dose should be started at a lower dose and doubled every 15 minutes until the patient is calm (maximum single bolus being 20 to 40 mg IV).
 Daily ECG to monitor that QTc remains below 550 msec in order to minimize theoretical risk for torsade de pointes.
Daily ECG to monitor that QTc remains below 550 msec in order to minimize theoretical risk for torsade de pointes.
 Potassium and magnesium should be replete to high normal daily to assist in minimization of QTc prolongation.
Potassium and magnesium should be replete to high normal daily to assist in minimization of QTc prolongation.
 Monitor for signs of extrapyramidal side effects, including akathisia.
Monitor for signs of extrapyramidal side effects, including akathisia.
 Quetiapine
Quetiapine
 Often helpful when administered in a standing fashion to patients with hyperactive delirium, or to those who are in a state of hyperarousal
Often helpful when administered in a standing fashion to patients with hyperactive delirium, or to those who are in a state of hyperarousal
 As a result of its α-1-antagonistic and histaminergic properties, quetiapine has stronger sedative effects than IV haloperidol.
As a result of its α-1-antagonistic and histaminergic properties, quetiapine has stronger sedative effects than IV haloperidol.
 Dosing range is generally 25 to 200 mg BID/TID with a maximum of 800 mg/d.
Dosing range is generally 25 to 200 mg BID/TID with a maximum of 800 mg/d.
 In dopamine antagonist-naïve patients, doses above 500 mg/d may cause anticholinergic excess (including decreased gastrointestinal motility and ileus) and this should be monitored for any patient on higher doses of quetiapine for greater than 72 hours.
In dopamine antagonist-naïve patients, doses above 500 mg/d may cause anticholinergic excess (including decreased gastrointestinal motility and ileus) and this should be monitored for any patient on higher doses of quetiapine for greater than 72 hours.
 Olanzapine
Olanzapine
 Due to its anticholinergic properties, olanzapine has limited usefulness in the delirious agitated ICU patient at dosages above 30 mg/d.
Due to its anticholinergic properties, olanzapine has limited usefulness in the delirious agitated ICU patient at dosages above 30 mg/d.
 Dosing range is generally 2.5 to 20 mg and may be given every 4 hours as needed to maximum daily dose of 30 mg.
Dosing range is generally 2.5 to 20 mg and may be given every 4 hours as needed to maximum daily dose of 30 mg.
 Olanzapine-zydis SL is NOT absorbed orally, but rather is dissolved and swallowed, with absorption occurring in the stomach.
Olanzapine-zydis SL is NOT absorbed orally, but rather is dissolved and swallowed, with absorption occurring in the stomach.
 Alpha agonists
Alpha agonists
 Dexmedetomidine
Dexmedetomidine
 Multiple studies have shown that dexmedetomidine is an effective agent for the control of agitation, including for the use of alcohol withdrawal that develops in the ICU.
Multiple studies have shown that dexmedetomidine is an effective agent for the control of agitation, including for the use of alcohol withdrawal that develops in the ICU.
 Loading doses generally should be avoided for this purpose to avoid potential for bradycardia and hypotension.
Loading doses generally should be avoided for this purpose to avoid potential for bradycardia and hypotension.
 Hypotension is usually associated with hypovolemia and once corrected, rechallenge may be attempted.
Hypotension is usually associated with hypovolemia and once corrected, rechallenge may be attempted.
 While not reported in the literature, the patient should be monitored for QTc prolongation.
While not reported in the literature, the patient should be monitored for QTc prolongation.
 Clonidine
Clonidine
 Clonidine may be helpful as a second or third-line agent for the control of treatment-refractory agitation.
Clonidine may be helpful as a second or third-line agent for the control of treatment-refractory agitation.
 Its use may be higher yield in patients with GABA receptor withdrawal or autonomic storming from acute TBI (with resulting diaphoresis, tachycardia, and hypertension).
Its use may be higher yield in patients with GABA receptor withdrawal or autonomic storming from acute TBI (with resulting diaphoresis, tachycardia, and hypertension).
 Other agents
Other agents
 Valproate
Valproate
 Intravenous and enteral valproate may be a second-line agent for the control of treatment-resistant agitation or for patients with prolonged QTc (> 600 msec) in which use of dopamine antagonists may be problematic, or for patients with acute TBI demonstrating impulsivity/disinhibition or other symptoms of a frontal network syndrome.
Intravenous and enteral valproate may be a second-line agent for the control of treatment-resistant agitation or for patients with prolonged QTc (> 600 msec) in which use of dopamine antagonists may be problematic, or for patients with acute TBI demonstrating impulsivity/disinhibition or other symptoms of a frontal network syndrome.
 Propranolol
Propranolol
 Use of propranolol (or other beta blockers) may be a reasonable second or third-line agent for heart rate control in patients with treatment-resistant agitation.
Use of propranolol (or other beta blockers) may be a reasonable second or third-line agent for heart rate control in patients with treatment-resistant agitation.
 It is commonly used for patients with traumatic brain injury with autonomic storming, and may be considered earlier in the treatment course.
It is commonly used for patients with traumatic brain injury with autonomic storming, and may be considered earlier in the treatment course.
 Use of propranolol can cause AMS in certain patients and its use should be followed closely.
Use of propranolol can cause AMS in certain patients and its use should be followed closely.
Outcomes
 In elderly delirious patients:
In elderly delirious patients:
 Less than half of elderly hospital inpatients with delirium are fully recovered at the time of discharge.
Less than half of elderly hospital inpatients with delirium are fully recovered at the time of discharge.
 20% to 75% of elderly delirious patients die during hospitalization and 25% within 6 months of onset.
20% to 75% of elderly delirious patients die during hospitalization and 25% within 6 months of onset.
 In non-ICU patients, the development of delirium is associated with
In non-ICU patients, the development of delirium is associated with
 In hospital mortality of 25% to 33%
In hospital mortality of 25% to 33%
 Prolonged hospital stay
Prolonged hospital stay
 Three times increase in the chance of discharge to a nursing facility
Three times increase in the chance of discharge to a nursing facility
 A prospective cohort study of mechanically ventilated patients (n = 275) demonstrated patients who developed delirium had a threefold increase in 6-month mortality rates compared with those who did not.
A prospective cohort study of mechanically ventilated patients (n = 275) demonstrated patients who developed delirium had a threefold increase in 6-month mortality rates compared with those who did not.
 Pisani and colleagues demonstrated a 10% increase in hazard of death (hazards ratio 1.10; 95% confidence interval, 1.02 to 1.18) up to 1 year after critical illness with each additional day of ICU stay.
Pisani and colleagues demonstrated a 10% increase in hazard of death (hazards ratio 1.10; 95% confidence interval, 1.02 to 1.18) up to 1 year after critical illness with each additional day of ICU stay.
 Findings were independent of age, severity of illness, comorbidities, coma, and exposure to psychoactive medications.
Findings were independent of age, severity of illness, comorbidities, coma, and exposure to psychoactive medications.
 Delirium in the ICU is associated with longer hospital stays.
Delirium in the ICU is associated with longer hospital stays.
 ICU survivors may have long-term brain dysfunction (e.g., functionally debilitating dementia-like illness), which appears to be predicted by delirium duration and is associated with increased rates of institutional placement.
ICU survivors may have long-term brain dysfunction (e.g., functionally debilitating dementia-like illness), which appears to be predicted by delirium duration and is associated with increased rates of institutional placement.
SUGGESTED READINGS
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision (DSM-IV-TR). Am Psychiatric Assoc. 2000.
Bamgbade OA. Dexmedetomidine for peri-operative sedation and analgesia in alcohol addiction. Anaesthesia. 2006;61:299-300.
Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive care delirium screening checklist: evaluation of a new screening tool. Intensive Care Med. 2001;27:859-864.
Cassem NH, Murray GM, Lafayette JM, Stern TA. Delirious patients. In: Stern TA, Fricchione GL, Cassem NH, Jellinek MS, Rosenbaum JF, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. Philadelphia, PA: Mosby; 2004:119-134.
Devlin JW, Roberts RJ, Fong JJ, et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Crit Care Med. 2010;38:419-427.
Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892-1900.
Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703-2710.
Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753-1762.
Fann JR. The epidemiology of delirium: a review of studies and methodological issues. Semin Clin Neuropsychiatry. 2000;5:64-74.
Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165:803-812.
Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87-98.
Maldonado JR. Pathoetiological model of delirium: a comprehensive understanding of the neurobiology of delirium and an evidence-based approach to prevention and treatment. Crit Care Clin. 2008;24: 789-856; ix.
Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180: 1092-1097.
Practice guideline for the treatment of patients with delirium. American psychiatric association. Am J Psychiatry. 1999;156:1-20.
Shehabi Y, Nakae H, Hammond N, Bass F, Nicholson L, Chen J. The effect of dexmedetomidine on agitation during weaning of mechanical ventilation in critically ill patients. Anaesth Intensive Care. 2010; 38:82-90.
Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: treating delirium in a critical care setting. Intensive Care Med. 2004;30:444-449.
Tang JF, Chen PL, Tang EJ, May TA, Stiver SI. Dexmedetomidine controls agitation and facilitates reliable, serial neurological examinations in a non-intubated patient with traumatic brain injury. Neurocritical Care. 2011;15:175-181.
9.2
Status Epilepticus
Introduction
 Status epilepticus (SE) is typically defined as repetitive seizures lasting 30 min or longer, without full return of consciousness between episodes.
Status epilepticus (SE) is typically defined as repetitive seizures lasting 30 min or longer, without full return of consciousness between episodes.
 Many recommend that seizure lasting > 5 min, or two seizures without a complete inter-ictal recovery should be defined as SE.
Many recommend that seizure lasting > 5 min, or two seizures without a complete inter-ictal recovery should be defined as SE.
 Important features include:
Important features include:
 SE is a manifestation of acute or chronic brain injury or toxic-metabolic insults.
SE is a manifestation of acute or chronic brain injury or toxic-metabolic insults.
 It is a medical emergency, and can result in irreversible neurological injury, pyrexia and rhabdomyolysis, systemic inflammatory response, respiratory failure, and circulatory collapse.
It is a medical emergency, and can result in irreversible neurological injury, pyrexia and rhabdomyolysis, systemic inflammatory response, respiratory failure, and circulatory collapse.
 Most cases of convulsive SE start as partial seizures that generalize.
Most cases of convulsive SE start as partial seizures that generalize.
 Cardiac injury due to massive catecholamine release, and an extreme metabolic stress associated with muscular convulsions can contribute to poor outcomes.
Cardiac injury due to massive catecholamine release, and an extreme metabolic stress associated with muscular convulsions can contribute to poor outcomes.
 Classification
Classification
 Generalized convulsive SE
Generalized convulsive SE
 Always associated with impaired consciousness
Always associated with impaired consciousness
 Tonic flexion of the axial muscles, arms, and legs is followed by extension, clenching of teeth, forced expiration, upward or lateral eye deviation and dilation of the pupils.
Tonic flexion of the axial muscles, arms, and legs is followed by extension, clenching of teeth, forced expiration, upward or lateral eye deviation and dilation of the pupils.
 The clonic phase is characterized by uninterrupted jerking or shivering.
The clonic phase is characterized by uninterrupted jerking or shivering.
 Muscle contractions may become reduced with increased duration of the seizures, despite continued electrical seizure activity.
Muscle contractions may become reduced with increased duration of the seizures, despite continued electrical seizure activity.
 Generalized tonic–clonic SE is associated with high risk of major complications and requires aggressive treatment.
Generalized tonic–clonic SE is associated with high risk of major complications and requires aggressive treatment.
 Complex partial (nonconvulsive) SE
Complex partial (nonconvulsive) SE
 Also associated with impaired consciousness, nonconvulsive SE is often preceded by psychosensory aura
Also associated with impaired consciousness, nonconvulsive SE is often preceded by psychosensory aura
 It often originates from mesial temporal or frontal lesions.
It often originates from mesial temporal or frontal lesions.
 Confusion and automatism may occur.
Confusion and automatism may occur.
 Simple partial SE
Simple partial SE
 Continuous or repeated focal motor seizures, sensory, autonomic or cognitive symptoms without impairment of consciousness
Continuous or repeated focal motor seizures, sensory, autonomic or cognitive symptoms without impairment of consciousness
 Myoclonic SE
Myoclonic SE
 Synchronous brief jerking of the limbs, face, or the diaphragm with altered consciousness
Synchronous brief jerking of the limbs, face, or the diaphragm with altered consciousness
 Myoclonic SE is described in patients with global cerebral ischemia, or after electrical injury, drug intoxication or decompression sickness.
Myoclonic SE is described in patients with global cerebral ischemia, or after electrical injury, drug intoxication or decompression sickness.
 It is commonly associated with severe cortical laminar necrosis with thalamic and spinal cord injuries.
It is commonly associated with severe cortical laminar necrosis with thalamic and spinal cord injuries.
 Nonconvulsive SE
Nonconvulsive SE
 Including absence SE (petit mal status), atypical absence (Lennox–Gastaut), atonic SE, and nonconvulsive SE as a sequel of partially treated generalized convulsive SE
Including absence SE (petit mal status), atypical absence (Lennox–Gastaut), atonic SE, and nonconvulsive SE as a sequel of partially treated generalized convulsive SE
Common Causes to Remember
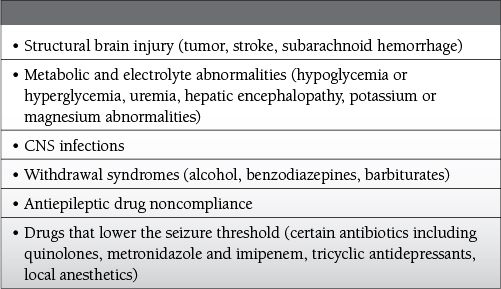
Epidemiology
 Affects 100 to 200,000 people in the United States per year
Affects 100 to 200,000 people in the United States per year
 Incidence is between 7 and 35 patients per ICU admission.
Incidence is between 7 and 35 patients per ICU admission.
 Refractory to first- and second-line drug therapy in 30% to 43% of patients.
Refractory to first- and second-line drug therapy in 30% to 43% of patients.
 Associated mortality is 20% to 80% (higher after anoxic brain injury).
Associated mortality is 20% to 80% (higher after anoxic brain injury).
Key Pathophysiology
 The pathophysiology of SE is not well understood but can be divided into predispositions, i.e., conditions that increase the likelihood of SE, and precipitants.
The pathophysiology of SE is not well understood but can be divided into predispositions, i.e., conditions that increase the likelihood of SE, and precipitants.
 Opening of ion channels that are coupled to excitatory amino acids (AMPA, NMDA, and metabotropic channels) can raise intracellular free calcium and lead to the generation of nitric oxide and oxygen free radicals, as well as phosphorylation of enzymes and receptor systems that result in an increased intracellular osmolality and neuronal swelling.
Opening of ion channels that are coupled to excitatory amino acids (AMPA, NMDA, and metabotropic channels) can raise intracellular free calcium and lead to the generation of nitric oxide and oxygen free radicals, as well as phosphorylation of enzymes and receptor systems that result in an increased intracellular osmolality and neuronal swelling.
 Failure of adenosine triphosphate production and activation of immediate—early genes with production of heat shock proteins have also been implicated.
Failure of adenosine triphosphate production and activation of immediate—early genes with production of heat shock proteins have also been implicated.
 Hypersynchrony of neuronal circuits, increased density of synapses and gap junctions, and formation of sprouting and abnormal connections have been reported in temporal lobe epilepsy.
Hypersynchrony of neuronal circuits, increased density of synapses and gap junctions, and formation of sprouting and abnormal connections have been reported in temporal lobe epilepsy.
 The increased neuronal activity during SE elevates demand for oxygen and glucose, which can be partly compensated for through an increase in cerebral blood flow, but as energy supplies become exhausted, cellular ion pumps start to fail, initiating a cascade of events that can lead to irreversible neurological injury.
The increased neuronal activity during SE elevates demand for oxygen and glucose, which can be partly compensated for through an increase in cerebral blood flow, but as energy supplies become exhausted, cellular ion pumps start to fail, initiating a cascade of events that can lead to irreversible neurological injury.
Differential Diagnosis
 Hypoglycemia, hypoxia, or other metabolic or infectious encephalopathies
Hypoglycemia, hypoxia, or other metabolic or infectious encephalopathies
 Structural brain injuries (e.g., pontine injuries)
Structural brain injuries (e.g., pontine injuries)
 Psychogenic nonepileptic seizures: bilateral motor movements with preserved consciousness and normal EEG recordings
Psychogenic nonepileptic seizures: bilateral motor movements with preserved consciousness and normal EEG recordings
 Creatinine kinase levels are not elevated.
Creatinine kinase levels are not elevated.
 Syncope: impairment of arousal and loss of postural tone caused by vasovagal reaction, cardiac dysrhythmias, or neurologic and psychiatric disease.
Syncope: impairment of arousal and loss of postural tone caused by vasovagal reaction, cardiac dysrhythmias, or neurologic and psychiatric disease.
 It is often self-limited and followed by complete recovery.
It is often self-limited and followed by complete recovery.
 Narcolepsy: a sleep disorder in which rapid eye movement sleep invades normal wakefulness.
Narcolepsy: a sleep disorder in which rapid eye movement sleep invades normal wakefulness.
Management and Treatment
 Initial treatment: includes airway control and support of respiration and circulation, as indicated
Initial treatment: includes airway control and support of respiration and circulation, as indicated
 Thiamine (100 mg IV) and glucose (50 mL of 50% dextrose) should be administered when etiology is unknown, or if hypoglycemia or alcohol withdrawal is suspected.
Thiamine (100 mg IV) and glucose (50 mL of 50% dextrose) should be administered when etiology is unknown, or if hypoglycemia or alcohol withdrawal is suspected.
 Correct obvious metabolic abnormalities (potassium, magnesium).
Correct obvious metabolic abnormalities (potassium, magnesium).
 Pharmacological treatment options include benzodiazepines, phenytoin, barbiturates and propofol.
Pharmacological treatment options include benzodiazepines, phenytoin, barbiturates and propofol.
 Treatment should ideally be administered intravenously, but if access is not readily available, diazepam can be given through alternative routes (rectal, intranasal, or buccal mucosa).
Treatment should ideally be administered intravenously, but if access is not readily available, diazepam can be given through alternative routes (rectal, intranasal, or buccal mucosa).
 First-line treatment
First-line treatment
 Lorazepam is considered first-line treatment for SE, despite its risk for respiratory depression.
Lorazepam is considered first-line treatment for SE, despite its risk for respiratory depression.
 It can be administered intravenously at the dose of 0.02 to 0.03 mg/kg (1 to 3 mg boluses) to a maximal adult dose of 10 mg (target dose 0.1 mg/kg).
It can be administered intravenously at the dose of 0.02 to 0.03 mg/kg (1 to 3 mg boluses) to a maximal adult dose of 10 mg (target dose 0.1 mg/kg).
 Lack of response after 5 min should be considered a failure.
Lack of response after 5 min should be considered a failure.
 Second-line treatment
Second-line treatment
 Phenytoin can be administered together with first-line treatment.
Phenytoin can be administered together with first-line treatment.
 Response rate is only 7% if first-line agent fails, and it may be reasonable to choose a more aggressive treatment with intravenous infusion of third-line agents.
Response rate is only 7% if first-line agent fails, and it may be reasonable to choose a more aggressive treatment with intravenous infusion of third-line agents.
 Phenytoin load of 18 to 20 mg/kg is administered at a maximum rate of 50 mg/min.
Phenytoin load of 18 to 20 mg/kg is administered at a maximum rate of 50 mg/min.
 EKG should be continuously monitored for arrhythmias, including bradycardia and heart block.
EKG should be continuously monitored for arrhythmias, including bradycardia and heart block.
 Fosphenytoin, a water-soluble form of phenytoin, lacks the propylene glycol vehicle that has been implicated in the hypotension and arrhythmias associated with phenytoin administration.
Fosphenytoin, a water-soluble form of phenytoin, lacks the propylene glycol vehicle that has been implicated in the hypotension and arrhythmias associated with phenytoin administration.
 Third-line treatment
Third-line treatment
 If seizure continues despite first- and second-line treatments, the patient should be admitted to an intensive care unit and phenobarbital administered at a loading dose of 15 to 20 mg/kg (100 mg/min).
If seizure continues despite first- and second-line treatments, the patient should be admitted to an intensive care unit and phenobarbital administered at a loading dose of 15 to 20 mg/kg (100 mg/min).
 Alternative treatments include levetiracetam (20 mg/kg) or sodium valproate (15 to 30 mg/kg).
Alternative treatments include levetiracetam (20 mg/kg) or sodium valproate (15 to 30 mg/kg).
 If the above treatment fails to stop the seizures, supportive care should be continued, patient’s airway controlled, and continuous infusion of midazolam (0.2 to 2 mg/kg/h), pentobarbital (0.25 to 2 mg/kg/h), or propofol (1 to 15 mg/kg/h) initiated to achieve burst suppression.
If the above treatment fails to stop the seizures, supportive care should be continued, patient’s airway controlled, and continuous infusion of midazolam (0.2 to 2 mg/kg/h), pentobarbital (0.25 to 2 mg/kg/h), or propofol (1 to 15 mg/kg/h) initiated to achieve burst suppression.
 Other drugs and management modalities
Other drugs and management modalities
 Ketamine (NMDA antagonist) and inhalational anesthetics are considered when above IV anesthetics fail.
Ketamine (NMDA antagonist) and inhalational anesthetics are considered when above IV anesthetics fail.
 Prolonged use of inhalational agents has been associated with T2 signal changes in thalamus and cerebellum.
Prolonged use of inhalational agents has been associated with T2 signal changes in thalamus and cerebellum.
 Lidocaine (3 to 4 mg/kg/h) stabilizes cell membranes and has been used with some success, but prolonged use has been associated with significant toxicity and exacerbation of seizures.
Lidocaine (3 to 4 mg/kg/h) stabilizes cell membranes and has been used with some success, but prolonged use has been associated with significant toxicity and exacerbation of seizures.
 Therapeutic hypothermia may be considered to reduce cerebral metabolic rate oxygen demands and excitotoxic transmission.
Therapeutic hypothermia may be considered to reduce cerebral metabolic rate oxygen demands and excitotoxic transmission.
 Vagal nerve or deep brain stimulation, plasmapheresis, high-dose steroids or IV gammaglobulin have been used (case reports), but there are currently no convincing data in support of these treatment modalities.
Vagal nerve or deep brain stimulation, plasmapheresis, high-dose steroids or IV gammaglobulin have been used (case reports), but there are currently no convincing data in support of these treatment modalities.
 Treatment of complications
Treatment of complications
 Hypervolemia and alkalinization of urine should be considered if rhabdomyolysis is suspected.
Hypervolemia and alkalinization of urine should be considered if rhabdomyolysis is suspected.
 Neuromuscular blocking agents may be considered, if seizure control is not obtained despite aggressive treatment, or if a patient does not tolerate increased sedation because of hypotension or arrhythmias.
Neuromuscular blocking agents may be considered, if seizure control is not obtained despite aggressive treatment, or if a patient does not tolerate increased sedation because of hypotension or arrhythmias.
 External cooling may be required to prevent hyperthermia if SE is not rapidly terminated.
External cooling may be required to prevent hyperthermia if SE is not rapidly terminated.
 Vasogenic cerebral edema may complicate SE, and can lead to an increased intracranial pressure requiring aggressive pharmacological management.
Vasogenic cerebral edema may complicate SE, and can lead to an increased intracranial pressure requiring aggressive pharmacological management.
 Steroids may be helpful.
Steroids may be helpful.
SUGGESTED READINGS
Bleck TP, Smith MC, Pierre-Louis JC, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illness. Crit Care Med. 1993;21:98-103.
Bleck TP. Management approaches to prolonged seizures and status epilepticus. Epilepsia. 1999;40(Suppl 1):S59-S63.
Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol. 2006;5(3):246-256.
Fountain NB, Lothman EW. Pathophysiology of status epilepticus. J Clin Neurophysiol. 1995;12:326-342.
Kaplan PW. Nonconvulsive status epilepticus. Semin Neurol. 1996;16:33-40.
Lorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12(4):316.
Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18(2):155.
Treiman DM. Generalized convulsive status epilepticus in the adult. Epilepsia. 1993;34(Suppl 1):S2-S11.
Trieman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans affairs status epilepticus cooperative study group. N Engl J Med. 1998;339(12): 792-798.
Wijdicks EFM, Sharbrough FW. New-onset seizures in critically ill patients. Neurology. 1993;43:1044-1044.
Depressive Disorders
Shamim H. Nejad and Christopher M. Celano
Introduction
There are four major types of mood episodes of which physicians should be aware when diagnosing mood disorders.
 Depressive
Depressive
 Characterized by depressed mood or anhedonia (the inability to experience pleasure) for most of the day nearly every day for at least 2 weeks and is accompanied by five or more of the following:
Characterized by depressed mood or anhedonia (the inability to experience pleasure) for most of the day nearly every day for at least 2 weeks and is accompanied by five or more of the following:
 Alterations in sleep
Alterations in sleep
 Lack of interest
Lack of interest
 Excessive feelings of guilt or worthlessness
Excessive feelings of guilt or worthlessness
 Low energy
Low energy
 Poor concentration
Poor concentration
 Change in appetite
Change in appetite
 Psychomotor agitation or retardation
Psychomotor agitation or retardation
 Suicidal thoughts or behaviors
Suicidal thoughts or behaviors
 Manic
Manic
 Characterized by elevated or irritable mood lasting for at least 1 week and accompanied by three or more (four or more if mood is irritable and not elevated) of the following:
Characterized by elevated or irritable mood lasting for at least 1 week and accompanied by three or more (four or more if mood is irritable and not elevated) of the following:
 Distractibility
Distractibility
 Decreased need for sleep
Decreased need for sleep
 Grandiosity or elevated self-esteem
Grandiosity or elevated self-esteem
 Racing thoughts
Racing thoughts
 Increased goal-directed activity or agitation
Increased goal-directed activity or agitation
 Talkativeness or pressured speech
Talkativeness or pressured speech
 Excessive engagement in pleasurable but risky activities
Excessive engagement in pleasurable but risky activities
 The symptoms lead to a marked disturbance in functioning or necessitate psychiatric hospitalization.
The symptoms lead to a marked disturbance in functioning or necessitate psychiatric hospitalization.
 Hypomanic
Hypomanic
 Characterized by elevated or irritable mood lasting for at least 4 days and accompanied by three or more manic symptoms (four or more if mood is irritable and not elevated)
Characterized by elevated or irritable mood lasting for at least 4 days and accompanied by three or more manic symptoms (four or more if mood is irritable and not elevated)
 These symptoms indicate a definite change in functioning that is noticed by others but is not severe enough to cause a marked impairment in social or occupational functioning, does not necessitate hospitalization, and is not accompanied by psychotic symptoms.
These symptoms indicate a definite change in functioning that is noticed by others but is not severe enough to cause a marked impairment in social or occupational functioning, does not necessitate hospitalization, and is not accompanied by psychotic symptoms.
 Mixed
Mixed
 Characterized by abnormal mood lasting for at least 1 week where patients meet criteria for both a major depressive and a manic episode concurrently
Characterized by abnormal mood lasting for at least 1 week where patients meet criteria for both a major depressive and a manic episode concurrently
 The mood change leads to marked impairment in functioning or necessitates hospitalization.
The mood change leads to marked impairment in functioning or necessitates hospitalization.
Definitions of Major Mood Disorders
 Major depressive disorder (MDD) is characterized by recurrent depressive episodes and by an absence of manic or hypomanic episodes.
Major depressive disorder (MDD) is characterized by recurrent depressive episodes and by an absence of manic or hypomanic episodes.
 Bipolar I disorder is characterized by at least one manic episode with or without depressive episodes.
Bipolar I disorder is characterized by at least one manic episode with or without depressive episodes.
 Bipolar II disorder is characterized by at least one depressive episode, at least one hypomanic episode, and no manic episodes.
Bipolar II disorder is characterized by at least one depressive episode, at least one hypomanic episode, and no manic episodes.
Definition of Dysthymic Disorder
 Depressed mood for most of the day for most days for at least 2 years, accompanied by two or more: (1) changes in appetite, (2) changes in sleep, (3) low energy, (4) poor self-esteem, (5) poor concentration, and (6) hopelessness.
Depressed mood for most of the day for most days for at least 2 years, accompanied by two or more: (1) changes in appetite, (2) changes in sleep, (3) low energy, (4) poor self-esteem, (5) poor concentration, and (6) hopelessness.
 These patients have no history of depressive episodes (in the first 2 years of these symptoms) and have not been free from symptoms for more than 2 months during the 2-year period.
These patients have no history of depressive episodes (in the first 2 years of these symptoms) and have not been free from symptoms for more than 2 months during the 2-year period.
Definition of Adjustment Disorder
 Clinically significant, emotional, and behavioral symptoms in response to an identifiable stressor occurring within 3 months of the onset of the stressor
Clinically significant, emotional, and behavioral symptoms in response to an identifiable stressor occurring within 3 months of the onset of the stressor
 These symptoms are excessive to what would be expected or significantly impact patient functioning and must resolve within 6 months of the termination of the stressor.
These symptoms are excessive to what would be expected or significantly impact patient functioning and must resolve within 6 months of the termination of the stressor.
It is important to differentiate MDD from bipolar and adjustment disorders, as treatment and prognosis differ significantly among these disorders.
Other Affective States Frequently Seen in the Intensive Care Unit (ICU)
 Apathy: indifference
Apathy: indifference
 Abulia: a lack of motivation that is more severe than apathy.
Abulia: a lack of motivation that is more severe than apathy.
 These affective/motivational states frequently can be seen in patients with delirium or other medical disorders impacting psychiatric function.
These affective/motivational states frequently can be seen in patients with delirium or other medical disorders impacting psychiatric function.
Common Causes to Remember
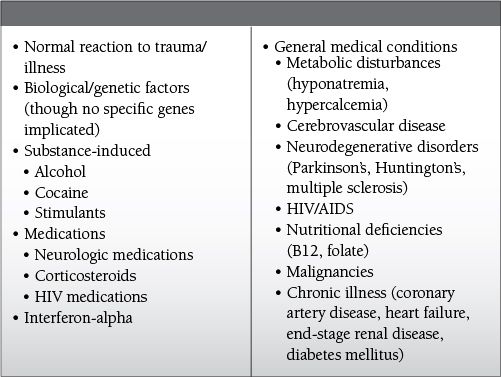
Epidemiology
 Lifetime prevalence of MDD in the general population: 13.3% to 17.1%
Lifetime prevalence of MDD in the general population: 13.3% to 17.1%
 Prevalence of depression in the ICU population
Prevalence of depression in the ICU population
 Very few studies have evaluated the prevalence of depression in patients admitted to the ICU, likely due to the fact that many symptoms of depression are experienced in the setting of acute illness even in the absence of a primary psychiatric disorder.
Very few studies have evaluated the prevalence of depression in patients admitted to the ICU, likely due to the fact that many symptoms of depression are experienced in the setting of acute illness even in the absence of a primary psychiatric disorder.
 However, patients admitted to the ICU are at high risk for depression in the months following discharge.
However, patients admitted to the ICU are at high risk for depression in the months following discharge.
 10% to 30% of patients may suffer from clinically significant depressive symptoms by 1 year post-discharge, and approximately 12% may have MDD.
10% to 30% of patients may suffer from clinically significant depressive symptoms by 1 year post-discharge, and approximately 12% may have MDD.
 One study suggested that post-discharge depressive symptoms are more likely to occur in patients initially admitted to the ICU for surgical reasons (compared to medical and trauma admissions).
One study suggested that post-discharge depressive symptoms are more likely to occur in patients initially admitted to the ICU for surgical reasons (compared to medical and trauma admissions).
 In-hospital psychosis and low levels of optimism have been correlated with post-discharge depression in some studies.
In-hospital psychosis and low levels of optimism have been correlated with post-discharge depression in some studies.
Key Pathophysiology
 The underlying etiology of MDD is multifactorial and likely involves a genetic predisposition accompanied by environmental stressors.
The underlying etiology of MDD is multifactorial and likely involves a genetic predisposition accompanied by environmental stressors.
 Several biological factors have been identified that may contribute to MDD.
Several biological factors have been identified that may contribute to MDD.
 Abnormalities in biogenic amines
Abnormalities in biogenic amines
 Decreased activity of serotonin, norepinephrine, and dopamine all have been implicated in the pathogenesis of some of the symptoms of MDD.
Decreased activity of serotonin, norepinephrine, and dopamine all have been implicated in the pathogenesis of some of the symptoms of MDD.
 Medications that increase or enhance dopaminergic, serotonergic, and noradrenergic neurotransmission frequently have antidepressant effects.
Medications that increase or enhance dopaminergic, serotonergic, and noradrenergic neurotransmission frequently have antidepressant effects.
 Abnormalities in the hypothalamic-pituitary-adrenal axis
Abnormalities in the hypothalamic-pituitary-adrenal axis
 Hypersecretion of cortisol has been associated with MDD.
Hypersecretion of cortisol has been associated with MDD.
 Evidence for this comes from findings that many patients with MDD fail to suppress cortisol production when given a dose of dexamethasone.
Evidence for this comes from findings that many patients with MDD fail to suppress cortisol production when given a dose of dexamethasone.
Differential Diagnosis for MDD
 Bipolar disorder (see above)
Bipolar disorder (see above)
 Dysthymic disorder (see above)
Dysthymic disorder (see above)
 Adjustment disorder (see above)
Adjustment disorder (see above)
 Schizoaffective disorder—characterized by a history of psychotic symptoms (delusions, hallucinations, abnormal thought process) in the absence of depressive symptoms (see chapter on psychotic disorders)
Schizoaffective disorder—characterized by a history of psychotic symptoms (delusions, hallucinations, abnormal thought process) in the absence of depressive symptoms (see chapter on psychotic disorders)
 Delirium
Delirium
 A disorder of disturbed consciousness with an acute to subacute (hours to days) onset which is characterized by
A disorder of disturbed consciousness with an acute to subacute (hours to days) onset which is characterized by
 Inattention
Inattention
 Perceptual disturbances
Perceptual disturbances
 Cognitive changes (disorientation, memory deficits, language disturbance)
Cognitive changes (disorientation, memory deficits, language disturbance)
 The patient’s level of consciousness tends to fluctuate over the course of the day
The patient’s level of consciousness tends to fluctuate over the course of the day
 Delirium is the direct physiological consequence of a medical illness.
Delirium is the direct physiological consequence of a medical illness.
 Patients with hypoactive delirium (i.e., those patients without significant agitation but with greater degrees of withdrawal and sedation) often are mistaken for being depressed.
Patients with hypoactive delirium (i.e., those patients without significant agitation but with greater degrees of withdrawal and sedation) often are mistaken for being depressed.
 It is important to remember, however, that attention and orientation remain normal in depressed patients, distinguishing them from those with hypoactive delirium.
It is important to remember, however, that attention and orientation remain normal in depressed patients, distinguishing them from those with hypoactive delirium.
 Dementia
Dementia
 A disorder with a gradual onset and progressive course (months to years) which is characterized by
A disorder with a gradual onset and progressive course (months to years) which is characterized by
 Memory impairment
Memory impairment
 Aphasia (language abnormalities)
Aphasia (language abnormalities)
 Apraxia (motor abnormalities)
Apraxia (motor abnormalities)
 Agnosia (inability to recognize and identify objects)
Agnosia (inability to recognize and identify objects)
 Executive dysfunction (poor planning, sequencing, etc.)
Executive dysfunction (poor planning, sequencing, etc.)
 Differentiated from depression by its prominent cognitive deficits, its gradual onset, and its progressive course.
Differentiated from depression by its prominent cognitive deficits, its gradual onset, and its progressive course.
 Substance-induced mood disorder
Substance-induced mood disorder
 Common in patients who are intoxicated or withdrawing from substances; however, they can persist for several weeks after withdrawal is complete.
Common in patients who are intoxicated or withdrawing from substances; however, they can persist for several weeks after withdrawal is complete.
 Common causes from illicit drugs
Common causes from illicit drugs
 Withdrawal from cocaine
Withdrawal from cocaine
 Withdrawal from methamphetamine or other stimulants
Withdrawal from methamphetamine or other stimulants
 Intoxication with or withdrawal from alcohol
Intoxication with or withdrawal from alcohol
 Intoxication/withdrawal from opioid medications
Intoxication/withdrawal from opioid medications
 Prescribed medications that have been associated with depression:
Prescribed medications that have been associated with depression:
 Neurologic medications: benzodiazepines, vigabatrin, topiramate, flunarizine
Neurologic medications: benzodiazepines, vigabatrin, topiramate, flunarizine
 Corticosteroids
Corticosteroids
 Anti-malarial medications: mefloquine
Anti-malarial medications: mefloquine
 Human immunodeficiency virus medications: efavirenz
Human immunodeficiency virus medications: efavirenz
 Hepatitis C virus medications: interferon-alpha
Hepatitis C virus medications: interferon-alpha
 Correlation between depressive symptoms and the initiation or withdrawal of a medication/substance is useful to determine the likelihood that the depressive symptoms are related to the substance.
Correlation between depressive symptoms and the initiation or withdrawal of a medication/substance is useful to determine the likelihood that the depressive symptoms are related to the substance.
 Mood disorder secondary to a general medical condition
Mood disorder secondary to a general medical condition
 Endocrine disorders: hypothyroidism, adrenal disorders
Endocrine disorders: hypothyroidism, adrenal disorders
 Nutritional deficiencies (vitamin B12, folate, niacin)
Nutritional deficiencies (vitamin B12, folate, niacin)
 Metabolic abnormalities (hyponatremia, hypercalcemia)
Metabolic abnormalities (hyponatremia, hypercalcemia)
 Anemia
Anemia
 Chronic illness
Chronic illness
 Malignancy (e.g., pancreatic cancer)
Malignancy (e.g., pancreatic cancer)
 Cardiovascular disease (coronary artery disease, congestive heart failure)
Cardiovascular disease (coronary artery disease, congestive heart failure)
 Diabetes mellitus
Diabetes mellitus
 End-stage renal disease
End-stage renal disease
 HIV
HIV
 Neurologic disease
Neurologic disease
 Cerebrovascular disease—most commonly involving the L hemisphere in prefrontal cortex or basal ganglia
Cerebrovascular disease—most commonly involving the L hemisphere in prefrontal cortex or basal ganglia
 Parkinson’s disease
Parkinson’s disease
 Huntington’s disease
Huntington’s disease
 Multiple sclerosis
Multiple sclerosis
Initial Laboratory Workup
 First-line: basic metabolic panel, complete blood count, B12, folate, thyroid stimulating hormone (TSH).
First-line: basic metabolic panel, complete blood count, B12, folate, thyroid stimulating hormone (TSH).
 Second-line: liver function tests, head imaging, HIV
Second-line: liver function tests, head imaging, HIV
Management and Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


