Edited by Peter Cameron Jonathan Knott Fever is a common presenting symptom to the emergency department (ED); about 5% of patients give fever as the reason for their visit. Most patients with fever have symptoms and signs that indicate the site or region of infection. A prospective study of patients aged 16 years or older who presented to an ED with fever≥37.9°C found that 85% had localizing symptoms and signs that suggested or identified a source of fever and 15% had unexplained fever after the history and examination [1]. Fever with no localizing symptoms or signs at presentation is often seen in the first day or two of the illness. Many patients with such a problem will ultimately prove to have self-limiting viral infections, but others will have non-viral infections requiring treatment. Among this latter group are illnesses that may be serious and even rapidly fatal. Over one-third of patients who have fever for more than a few days with no localizing symptoms and signs are likely to have a bacterial infection [1,2]. If no cause is found in an adult with fever present for over 3 days, there is a good chance the patient will have a bacterial infection that needs treatment. Over half of these infections are likely to be in the respiratory or urinary tracts [1]. The most important task in the ED for febrile patients without localizing features is not to miss early bacterial meningitis, bacteraemia, such as meningococcaemia and early staphylococcal and streptococcal toxic shock syndromes. The management of febrile patients varies according to the severity, duration and tempo of the illness, the type of patient and the epidemiological setting. Although the steps in management of a febrile patient in the ED, listed below, may be set out in a sequential manner, in reality the mental processes involved occur simultaneously by the bedside. The first step in managing febrile patients is to identify those in need of immediate resuscitation, urgent investigations and empirical therapy. The presence of any of the following features justifies immediate intervention: shock, coma/stupor, cyanosis, profound dyspnoea, continuous seizures and severe dehydration. Having excluded those who need urgent intervention, the doctor has more time to attempt a diagnosis. The history and physical examination are usually sufficient to localize the source of community-acquired fever in most cases, especially if the illness has been present for several days. A precise history remains the key to diagnosis of a febrile illness. An inability to give a history and to think clearly is a sign of potential sepsis. An abrupt onset of fever, particularly when accompanied by chills or rigors and generalized aches, is highly suggestive of an infective illness. Localizing symptoms, their evolution and relative severity, help to identify the site of infection; localized pain is particularly valuable in this way. The severity and the course of the illness can be assessed by the patient’s ability to work, to be up and about, to eat and sleep and the amount of analgesics taken. Underlying diseases predispose patients to infection at certain sites or caused by certain specific organisms. Knowledge of any defects in the immune system is similarly helpful. For example, asplenic patients are more prone to overwhelming pneumococcal septicaemia and renal transplant patients to Listeria meningitis. A past history of infectious diseases, particularly if properly documented, may be useful in excluding infections such as measles and hepatitis. Recent operations, accidents and injuries and medications taken may be the direct cause of the illness (e.g. drug fever or rash from co-trimoxazole, ampicillin) or may affect the resistance of the patient, predisposing to certain infections. Concurrent menstruation raises the possibility of toxic shock syndrome. Information on occupation, exposure to animals, hobbies, risk factors for blood-borne viruses and travel overseas or to rural areas may suggest certain specific infections, e.g. leptospirosis, acute HIV infection, hepatitis C, malaria, etc. This information is useful in the diagnosis of problems such as meningococcal infection, viral exanthema, respiratory infection, diarrhoea, and zoonoses. Physical examination in the febrile patient serves two purposes: to assess the severity of the illness and to find a site of infection. Bedside assessment of severity and ‘toxicity’ based on intuitive judgement is frequently wrong and many patients with severe bacterial infections do not appear obviously ill or toxic. Physical examination may yield a diagnosis in a febrile patient who has not complained of any localizing symptoms. A checklist of special areas to be examined is useful. If no diagnosis is forthcoming after the first two steps, the next task is to identify the ‘at-risk’ patient who may not appear overtly ill but who, nonetheless, requires medical intervention. This applies particularly to those with treatable diseases that can progress rapidly, such as bacterial meningitis, bacteraemia and toxic shock syndromes. Four sets of pointers are helpful in identifying these ‘at-risk’ patients: the type of patient (host characteristics), exposure history, the nature of the non-specific symptoms and how rapidly the illness evolves. Clinical manifestations of infections are often subtle or non-specific in young children, the elderly and the immunocompromised. The threshold for intervention in these patients should be lowered. The issue of fever in children is not addressed in this chapter. Elderly patients with infections often do not mount much of a febrile response and fever may be absent in 20–30% of these patients [3]. Infectious diseases in the elderly, as in the very young, often present with non-specific or atypical symptoms and signs and may progress rapidly [4]. In adult patients with unexplained fever, up to one-third may have bacteraemia or focal bacterial infection. This proportion is even higher in those over the age of 50 [1]. In the elderly, a fever>38°C indicates a possible serious infection [5] and is associated with increasing risk of death [6]. The urinary tract is the most frequent site of infection and source of bacteraemia; symptoms of urinary tract infection are frequently absent in the elderly. The respiratory tract is the next most common site of infection; fever and malaise may be the only clues of pneumonia in the elderly. Urinalysis and chest X-ray will identify about half of occult infections [1]. An unexplained fever in a person over the age of 50 should be regarded as being caused by a bacterial infection until proved otherwise and is generally an indication for admission to hospital. Alcoholic patients present with multiple problems, many of which cause fever. Most are caused by infections, the commonest of which is pneumonia. Multiple infections may occur at the same time [7]. Non-infectious causes of fever frequently coexist with infections and conditions such as subarachnoid haemorrhage, alcoholic withdrawal and alcoholic hepatitis and require admission. The initial history and physical examination in the alcoholic may be unreliable and diagnosis may be difficult. Alcoholic patients with fever for which no obvious cause is found should be admitted to hospital for investigations and observation. The risk of injecting drug users acquiring serious or unusual infections is high through repeated self-injection with non-sterile illicit substances, the use of contaminated needles and syringes and poor attention to skin cleansing prior to injections [8]. Many intravenous drug users presenting with fever have a serious infection. Some have obvious focal infections, such as cellulitis and pneumonia. Others present simply with fever and the presence of bacteraemia and endocarditis must be suspected. Clinical assessment cannot differentiate trivial from potentially serious conditions in these patients [8]. A history of chills, rigors and sweats strongly suggest the presence of a transient or ongoing bacteraemia. Back pain may be a subtle symptom of endocarditis or vertebral osteomyelitis. It is difficult to distinguish the patient with endocarditis from other drug users with fever due to another cause. Hospitalization of febrile injecting drug users would be prudent if 24-hour follow up is not possible. Intravenous drug use in the previous 5 days is a predictor of occult major infection and is an indication for admission to hospital [9]. Diabetic patients are more prone to developing certain bacterial infections [1]. A diabetic patient with an unexplained fever is more likely to have an occult bacterial infection than a non-diabetic patient. In general, an insulin-dependent diabetic patient, especially if aged over 50, with fever and no obvious source of infection, should be investigated and preferably admitted. Febrile neutropaenic patients (absolute neutrophil count<500/μL or<1000/μL and falling rapidly) must be hospitalized regardless of their clinical appearance. Infections may become fulminant within hours in these patients and the clinical manifestations of their infective illnesses are frequently modified by the underlying disease, therapy received and coexisting problems. Splenectomized patients with fever must be very carefully assessed because of their increased risk of overwhelming bacterial infection. If the fever cannot be readily explained, admission for intravenous antibiotics is usually indicated. Fever in transplant patients (renal, hepatic or cardiac) and those with HIV infection is not an absolute indication for admission, but the threshold of intervention should be considerably lowered and they are best assessed by their usual treating doctors. Patients recently discharged from hospital may have hospital-acquired infections or infections caused by multiresistant organisms. Recent operations or procedures may be a clue to the site of infection. Returned travellers or overseas visitors may have diseases such as malaria and typhoid fever that need early diagnosis and treatment. Any fever in a traveller returned from a malaria-endemic area should be regarded as due to malaria until proved otherwise. Influenza in febrile returned travellers is a concern to EDs worldwide. Outbreaks of avian influenza occur periodically in bird populations throughout Asia. Although the virus does not typically infect humans, direct bird-to-human transmission of H5N1 influenza has been documented. The virus is highly pathogenic and the mortality of the disease is high. Travellers acquiring influenza overseas may also introduce this infection. Most cases occur within 2–4 days after exposure, but incubation is as long as 8 days. Suspected influenza infection requires isolation and respiratory precautions. The peak season is generally during the winter months, but can vary, especially in the tropics [10]. Although rare, viral haemorrhagic fever in returned travellers represents a true medical emergency and a serious public health threat. Viral haemorrhagic fevers are caused by several distinct families of virus, including Ebola and Marburg, Lassa fever, the New World arenaviruses (Guanarito, Machupo, Junin, and Sabia) and Rift Valley fever and Crimean Congo haemorrhagic fever viruses. Most exist in Africa, the Middle East or South America. Although some types cause relatively mild illnesses, many can cause severe, life-threatening disease. Viral haemorrhagic fever should be considered in any febrile patient who has returned from an area in which viral haemorrhagic fever was endemic, especially if they have come into contact with blood or other body fluids from a person or animal infected with viral haemorrhagic fever or worked in a laboratory or animal facility handling viral haemorrhagic fever specimens. All these infections have incubation periods of up to 2–3 weeks, so it may be possible to exclude viral haemorrhagic fever on epidemiological grounds alone. Isolation measures should be instituted immediately in these persons [11]. Staff working in emergency departments should be aware of regional outbreaks of unusual pathogens. These are reported by State and National Departments of Health. Returning travellers who are unwell will commonly go directly to an emergency department and this may be a critical point to limit further spread. A contact history with animals, either at work or at home, is frequently the clue to a zoonosis, particularly if the illness is a perplexing fever of several days’ duration. The occurrence of multiple cases at work or at home should also make one suspect these infections early. Close contacts of patients with these infections have a high risk of acquiring the same infections. Early symptoms may be subtle and a high index of suspicion must be maintained. There are several non-specific clinical features whose presence should suggest the possibility of sepsis. These warrant careful scrutiny even when the patient does not appear toxic. They are by no means specific indicators of serious problems and there will be many false positives. However, ignoring them is frequently the cause of missed or delayed diagnosis of sepsis. Table 9.1.1 Clinical pointers: non-specific clinical features (‘alarm bells’) Severe pain in muscles, neck or back Impairment of conscious state Vomiting, especially in association with headache or abdominal pain Severe headache in the presence of a normal CSF Unexplained rash Jaundice Severe sore throat or dysphagia with a normal looking throat Repeated rigors Severe muscle pain, even in the absence of overt fever, may be an early symptom of meningococcaemia, staphylococcal or streptococcal bacteraemia. It is also a feature of myositis and necrotizing fasciitis. A change in conscious state may be the sole presenting manifestation of sepsis, especially in the elderly. Unexplained vomiting, especially in association with headache or abdominal pain, should raise concern. Vomiting without diarrhoea should not be attributed to a gastrointestinal infection. It is a common symptom of CNS infections and occult sepsis. This is especially important in a person who seldom gets headaches. Severe headache in a febrile patient with normal CSF should not be diagnosed as a viral infection; many focal infections, e.g. pneumonia and bacterial enteritis, may also present in this manner. CSF may be normal in cerebral abscess and in the prodromal phase of bacterial meningitis. An unexplained rash in a febrile patient should be regarded as meningococcaemia until proved otherwise, even in the absence of headache or CSF pleocytosis. Jaundice in the febrile patient is associated with a greatly increased risk of death, admission to ICU and prolonged hospital stay [6]. Jaundice in a febrile patient is unlikely to be due to viral hepatitis, but occurs in serious bacterial infections, such as bacteraemia, cholangitis, pyogenic liver abscess and malaria. Severe sore throat or dysphagia with a normal-looking throat is frequently the presenting symptom of Haemophilus influenzae epiglottitis in adults. Although repeated rigors may occur in some viral infections, they should generally be regarded as indicators of sepsis, in particular abscesses, bacteraemia, endocarditis, cholangitis and pyelonephritis. How rapidly the illness evolves is often an indication of its severity. Previously healthy individuals do not seek medical attention unless they are worried. Notice should be taken of any person seeking help within 24 hours of the onset of illness or a person whose illness appears to have progressed rapidly within 24–48 hours (e.g. from being up and about to being bedridden). Similarly, the patient who presents to the ED on more than one occasion over a 24–48-hour period warrants a careful work-up. Table 9.1.2 Clinical pointers: evolution of illness Those presenting early (<24 hours) Those presenting with rapidly evolving symptoms Patients presenting to ED on>1 occasion over a 24–48-hour period A major concern in the management of undifferentiated fever in adults is missing the diagnosis of meningococcal bacteraemia when the patient does not appear ill on presentation. There are a number of infections that must be treated rapidly to minimize morbidity and mortality (Table 9.1.3). With the exception of meningococcal bacteraemia, there are usually some clues in the history or physical examination. Table 9.1.3 Infections requiring urgent treatment Meningococcal infection is peculiar in its wide spectrum of severity and variable rate of progression in different individuals. It may be fulminant and cause death within 12 hours or it may assume a chronic form that goes on for weeks. When the patient presents with fever and a petechial rash, meningococcaemia can easily be suspected if one remembers the golden rule of medicine that ‘fever plus a petechial rash is meningococcaemia (or staphylococcal bacteraemia) until proved otherwise’. However, only 40% of meningococcal diseases present with a petechial rash. It is less well known that the early meningococcaemic rash may be macular, i.e. one that blanches with pressure. This is the basis of another golden rule in infectious disease: early meningococcal rash may resemble a non-specific viral rash. Rarely, meningococcal disease presents with symptoms and signs of a localized infection other than meningitis, e.g. pneumonia, pericarditis or urethritis. These presentations should not pose any management problems. The risk of missing the diagnosis increases markedly when the patient with meningococcal disease presents with fever and non-specific symptoms without a rash. Abrupt onset of fever and generalized aches may be due to influenza, but it could be due to meningococcaemia. It is prudent to single out meningococcal disease and ask oneself: could this patient have meningococcaemia? If in doubt, the safest course is to take cultures, give antibiotics and admit. Most febrile patients seen in the ED justify a fever work-up. Full blood examination is of limited use. White cell count (>15×109/L), marked left shift, neutropaenia or thrombocytopaenia are pointers to a possible bacteraemia or occult bacterial infections, but they may also be seen in viral infections [12]. Similarly, non-specific markers of inflammation, such as C-reactive protein and erythrocyte sedimentation rate, have not been shown to be useful in predicting outcomes for febrile patients in the ED [13]. Urinalysis and urine culture should be done in febrile adults over the age of 50 unless the pathology clearly lies in another body system. However, if the history does not suggest urinary sepsis and the dipstick urinalysis is normal, then urine cultures are usually negative [14]. A chest X -ray is usually indicated unless a definite diagnosis has been made, e.g. chickenpox, tonsillitis. Blood cultures should be done in anyone suspected of having bacteraemia, endocarditis or meningitis, in compromised patients with a fever, all febrile patients over the age of 50 and, possibly, in anyone with an unexplained high fever. It should be noted that only 5% of blood cultures in this setting will be positive and less than 2% will alter clinical management [15]. In general, a patient considered ‘sick enough’ to warrant blood cultures should be admitted to hospital or followed up within 24 hours. Patients who have any of the following features are in need of resuscitation, followed by work-up and admission: shock, coma/stupor, cyanosis, profound dyspnoea, continuous seizures and severe dehydration. With few exceptions, the following groups of febrile adults should be investigated and admitted: In general, there should be close liaison with the admitting unit and the issue of empirical therapy for septic patients should be discussed. For the dangerously ill, e.g. those with septic shock or bacterial meningitis, antibiotics should be commenced almost immediately. There is an increasing tendency to start antibiotics in the ED as soon as possible to reduce the length of hospital stay. Time to antibiotic therapy is used as a key performance indicator for the ED, e.g. for febrile immunocompromised patients. Patients who do not require intervention after the basic work-up in the ED are discharged home after a period of observation. Because of the time taken to interview the patient, perform investigations and wait for the results, the patient will usually have been observed for 1–2 hours and progression or lack of progression may be a help in deciding what to do. During observation one must be aware that the apparent improvement of the patient may be the result of pain relief or a fall in temperature due to antipyretics. Arrangement must be made for the patient to be reviewed by their general practitioner or at the hospital. This is an essential component of the care of a febrile patient seen in an ED. There is no easy way of detecting occult bacterial sepsis. The infectious process is a dynamic one and the doctor must maintain contact with the patient or family during the 24–72 hours following the initial visit. Patients with fever>39°C must be seen within 24 hours. Review by a doctor within 6–12 hours may be necessary in those who have had a lumbar puncture and is advisable in those who have had blood cultures taken. A verified phone number should be clearly recorded in the medical history. All febrile patients discharged from the ED should be encouraged to seek review if there is any adverse change to their condition. A patient re-presenting to the ED has provided an opportunity to ensure that they are being managed appropriately and to rectify any errors. Fever due to most common viral infections will resolve by about 4 days. Many other infections will be diagnosed when new symptoms or signs appear. If fever persists beyond 4–5 days without any localizing symptoms or signs, a less common infection or non-infective cause should be suspected and the patient should be thoroughly investigated. In this situation, the threshold of admission to hospital should be low. The establishment of ED short-stay units allows fast-track treatment and observation, usually for 24–48 hours, for carefully selected febrile patients who are not suitable for immediate discharge home. Andrew Singer Meningitis is an inflammation of the leptomeninges, the membranes that line the central nervous system, as well as the cerebrospinal fluid (CSF) in the subarachnoid space. It is usually the result of an infection, but can be due to an inflammatory response to a localized or systemic insult. Meningitis is usually classified according to the aetiology or location as bacterial, aseptic (viral, tuberculous, fungal or chemical) or spinal (where the infection specifically affects the spinal meninges). Bacterial meningitis is a serious cause of morbidity and mortality in all age groups. The causes vary according to age, as shown in Table 9.2.1. Neisseria meningitidis serogroups A and C tend to cause endemic cases of meningitis, especially in Aboriginal populations, whereas serogroup B is more commonly associated with epidemics [1]. There has been an increase in the incidence of penicillin-resistant Streptococcus pneumoniae, especially in children [2]. Table 9.2.1 Aseptic meningitis may be either due to an immune response to a systemic infection (usually viral) or to a chemical insult. Enteroviruses are the most common cause of meningitis, often in clusters of cases. Herpes viruses often cause meningitis as part of a more generalized infection of the brain (meningoencephalitis) or as part of an immune response to a systemic infection. A generalized viraemia may also cause aseptic meningitis, owing to an immune reaction without direct infection. Fungal causes of meningitis, especially that due to Cryptococcus neoformans, tend to occur in immunocompromised patients, such as those with HIV/AIDS or those on immunosuppressant medication or cancer chemotherapy. It can occur in immunocompetent individuals as well, particularly the elderly. Tuberculous meningitis is rare in industrialized countries, but can occur in all age groups. It tends to follow an insidious course, with a lack of classic signs and symptoms. Diagnosis is often difficult, owing to the low yield from CSF staining and the 4-week time frame required to culture the organism. Suspicion should be high in patients with immunocompromise or chronic illness. It tends to have a high mortality. Spinal meningitis is usually bacterial and due to direct spread from a localized infection in the spine. The epidemiology of meningitis is different for groups according to age, as well as immunocompetence: Initially, there is colonization of the infectious agent, commonly in the nasopharynx in the case of the enteroviruses and bacteria, such as meningococcus and Hib. Other infections may spread from already established foci, such as otitis media or sinusitis (e.g. pneumococcus). There is either haematogenous or local spread to the meninges and subarachnoid space, with inflammation of this area and the production of a purulent exudate approximately 2 hours after invasion of the area. The inflammatory response is initiated by bacterial subcapsular components, such as lipoteichoic acid in S. pneumoniae, a lipo-oligosaccharide in H. influenzae and other Gram-negative endotoxins. These substances stimulate the release of cytokines, such as interleukin-1 and -6, tumour necrosis factor (TNF) and arachidonic acid metabolites, as well as the complement cascade. There is a subsequent increase in neutrophil and platelet activity, with increased permeability of the blood–brain barrier. This response is often worse after the initial destruction of bacteria by antibiotics. If left untreated, fibrosis of the meninges may occur. In viral and aseptic meningitis, there is a more limited inflammatory response, with mild-to-moderate infiltration of lymphocytes. In the more chronic causes, such as fungi or tuberculosis, the exudate is fibrinous, the main cells being a mixture of lymphocytes, monocytes/macrophages and plasma cells. The base of the brain is most commonly affected. There are some differences in the history with different causes of meningitis, which may allow an early differential diagnosis to be made. There are no pathognomonic single symptoms or signs for meningitis, so a high index of suspicion is necessary. The combination of fever, headache, meningism and mental obtundation is found in approximately 85% of cases of bacterial meningitis [9]. It is also a common pattern in viral or aseptic meningitis, where obtundation is less of a feature. In fungal or tuberculous meningitis, these symptoms are much less common (less than 40% of cases of cryptococcal meningitis). Elderly patients or those who have had recent neurosurgery may present with subtle or mild symptoms and lack a fever [10]. The headache is usually severe and unrelenting. It may be either global or located in a specific area. The main symptoms of meningism are nuchal rigidity (neck stiffness) and photophobia. The nuchal rigidity is something more than merely pain on movement of the neck. It is clinically important when the patient complains of a painful restriction of movement in the sagittal plane (i.e. forwards and backwards only). Up to 35% of cases have associated nausea and vomiting. As a general rule, the height of the fever is a poor indication of the possible cause, although the fever may often only be mild in tuberculous or fungal meningitis or in bacterial meningitis that has been partially treated by antibiotics. The spectrum of mental obtundation can range from mild confusion, to bizarre behaviour, delirium or coma. The severity of obtundation is a good indication of the severity of the illness. Focal neurological signs occur in around 10–20% of cases of bacterial meningitis, but are also associated with cerebral mass lesions, such as toxoplasmosis or brain abscess. They are also a feature of tuberculous meningitis. Seizures are relatively uncommon (13–30%), but may occasionally be the only sign of meningitis if the patient has been partially treated with oral antibiotics. There may also be associated systemic symptoms. Myalgias and arthralgias are often associated with viral causes, but may also be the sole presenting symptom in meningococcal meningitis. HIV/AIDS patients may show stigmata associated with that disease. The course of the illness may also indicate the cause. Meningococcal or pneumococcal meningitis is often characterized by a rapid, fulminating course, often going from initial symptoms to death over an interval of hours. Viral causes tend to be a slower course over days. Fungal or tuberculous meningitis shows a more chronic course over days to weeks, with milder symptoms. Risk factors for meningitis include the extremes of age, pre-existing sinusitis or otitis media, recent neurosurgery, CSF shunts, splenectomy, immunological compromise and chronic diseases such as alcoholism, cancer, connective tissue disorders, chronic renal failure and hepatic cirrhosis. The physical examination will often reflect symptoms elicited in the history, with fever, physical evidence of meningism, stigmata of AIDS, etc. As stated above, neck stiffness is only clinically significant when it occurs in the sagittal plane. There will be a restriction of both passive and active movement. Other tests to elicit meningism include Kernig’s sign and Brudzinski’s sign, although these are only present in 50% of adult cases of bacterial meningitis. Kernig’s sign is elicited by attempting to extend the knee of a leg that has been flexed at the hip with the patient lying supine and the other leg flat on the bed. The sign is positive if the knee cannot be fully extended due to spasm in the hamstrings. The test can be falsely positive in patients with shortening of the hamstrings or other problems involving the legs or lumbar spine. In Brudzinski’s sign, flexing the head causes the thighs and knees to also flex. It can also be tested in children by the inability to touch the nose with the flexed hips and knees in the sitting position. These are both late signs. Focal neurological signs should be a cause for concern, as they can indicate a poor prognosis. Papilloedema is rare and late, as is a bulging fontanelle in infants and should alert one to alternative diagnoses. A rash, often starting as a macular or petechial rash on the limbs, is seen in sepsis due to N. meningitidis and S. pneumoniae. A petechial rash is a particularly serious sign and is an indication to start antibiotics immediately. A maculopapular rash is also a feature of viral causes. A CSF sample via a lumbar puncture (LP) is an important source of information for making the diagnosis and determining the likely aetiology and treatment. As the procedure may be time-consuming, treatment should not be delayed if there will be more than a 20-minute delay before the lumbar puncture and there is a reasonable clinical suspicion that a bacterial cause is present. Blood cultures should be taken prior to the administration of antibiotics. The main features to note during lumbar puncture are the opening pressure and the physical appearance of the CSF. The sample should be sent for Gram staining, culture, sensitivities, polymerase chain reaction (PCR) analysis for bacteria and Herpes simplex virus, a cell count and protein and glucose levels. If fungal meningitis is suspected, an India-ink stain and cryptococcal antigen screen should be requested. If tuberculous meningitis is suspected, multiple 5 mL samples of CSF will be required to increase the likelihood of a positive result. If there has been prior administration of antibiotics, a bacterial antigen screen should also be requested. Turbid CSF is indicative of a significant number of pus cells and is an indication for immediate administration of antibiotics. The patient should usually rest supine for a few hours after the procedure to prevent a worsening of the headache. This has been known to occur up to 24 hours following the procedure. The evidence for the benefits of enforced rest after lumbar puncture is equivocal. The pattern of cell counts and glucose and protein levels is shown in Table 9.2.2. This can act as a guide only and the clinician needs to be guided by the complete clinical picture. Table 9.2.2 Expected CSF values in meningitis A leucocyte count (WCC) of more than 1000/μL with a predominantly neutrophilic pleocytosis is considered positive for bacterial meningitis. Ten per cent of cases, especially early in the course of the illness, may have a predominance of lymphocytes. As a general rule, bacterial meningitis is characterized by a raised CSF protein and a low CSF glucose level. The ratio of CSF to serum glucose levels is also lowered. The combination of CSF glucose<1.9 mmol/L, CSF to serum glucose ratio<0.23, CSF protein>2.2 g/L and either a total WCC>2000/μL or a neutrophil count of>1180/μL has been shown to have a 99% certainty of diagnosing bacterial meningitis [11]. Aseptic meningitis will often have cell counts near the normal range. This does not exclude infection with less common agents, such as herpes viruses or L. monocytogenes. CT scanning of the brain is indicated as a prelude to lumbar puncture in the presence of focal neurological signs, mental obtundation or abnormal posturing. It must be noted though, that a normal CT does not exclude the risk of cerebral herniation in bacterial meningitis [12] and, therefore, those with the above signs should have lumbar puncture delayed until they are conscious and stable. Apart from microscopy and culture of CSF, there are a number of other methods that may allow the causative organism to be identified. In cases where a petechial rash is present, Gram staining or culture from some of the skin lesions may yield the causative organism. This has a reported sensitivity of 30–70%. Throat swabs are useful in identifying a bacterial cause spread by nasopharyngeal carriage and should be performed in a case of suspected bacterial meningitis. This potentially allows identification of the causative organism and even the serotype for organisms, such as meningococcus. The test can be performed on CSF or EDTA blood samples and may remain positive for up to 72 hours after the commencement of antibiotics. In CSF, the reported sensitivity is 89% with a specificity of 100% and in blood a sensitivity of 81% with a specificity of 97% [13]. Tests to detect IgM to specific organisms are available for meningococcus and some viruses. For meningococcus, the test has a sensitivity and specificity of 97% and 95%, but is only reliable in adults and children over 4 years old and takes 5–7 days after onset of the illness to reach diagnostic levels. Latex agglutination, immunoelectrophoresis or radioimmunoassay techniques can be used to screen for antigens from S. pneumoniae, Hib, group B streptococcus (S. agalactiae), Escherichia coli K1, N. meningitidis and C. neoformans. The tests can be performed on serum, CSF or urine. Serum or urine samples tend to allow greater sensitivities (around 96–99%) than CSF (82–99%). The test is no more sensitive in untreated cases than either a positive Gram-stain or the presence of CSF pleocytosis [14]. The main purpose of antigenic studies is in allowing rapid identification of the causative organism in cases confirmed by the CSF findings or in cases where partial treatment with antibiotics renders the CSF sterile on culture. In many laboratories, these tests have been superseded by PCR methods. Full blood count (FBC), urea and electrolyte counts (UEC), blood cultures, erythrocyte sedimentation rate (ESR) and a throat swab can assist in building an overall picture. Blood cultures should be taken prior to parenteral antibiotics, especially in patients where lumbar puncture has been delayed. One study found that blood cultures grew the causative organism in 86% of proven cases of bacterial meningitis and that the combination of blood culture, CSF Gram staining and antigen testing identified the cause in 92% of cases [15]. Management depends on the likely causative agents, as well as the severity of the illness. Patients should rest in bed, particularly following a lumbar puncture. A quiet, darkened room will be beneficial to those with headache or photophobia. Simple analgesics may be used to treat the headache, with or without codeine. Opiates may be required in severe headache. Sedation may be necessary if the patient is very agitated or delirious. Suitable drugs are diazepam 5–10 mg IV or midazolam 2–10 mg IV or IM, with or without the addition of an antipsychotic, such as haloperidol 5–20 mg IV or IM, or chlorpromazine 12.5–50 mg IV or IM. Seizures should be treated appropriately, initially with a benzodiazepine, then maintenance with phenytoin or phenobarbitone. Meningitis can occasionally be associated with status epilepticus, which should be treated in the standard way. Patients with raised intracranial pressure may need pressure monitoring and measures to reduce the pressure, such as nursing the patient 30° head up and the administration of hyperosmotic agents, such as mannitol. Hyperventilation is controversial as it may reduce intracerebral pressure at the expense of reduced cerebral perfusion. Obstructive hydrocephalus requires appropriate neurosurgical treatment with CSF shunting. If septic shock has intervened, it should be treated in the usual way, with IV fluids and inotropes. The choice of antimicrobial agent will be determined by the likely causative organism and is therefore determined primarily by age and immune status. It is important that antibiotic therapy is not delayed by investigations such as lumbar puncture or CT and should be administered as soon as the diagnosis is made. Table 9.2.3 shows the recommended choice of antimicrobial for different situations and organisms. Table 9.2.4 shows the recommended dosage of each. As a general rule, the combination of a third-generation cephalosporin and benzylpenicillin will cover most organisms in all age groups. It is important to note that there is emerging resistance to penicillins in S. pneumoniae (currently 7.6% of isolates in Australia). If Gram-positive diplococci are found or S. pneumoniae is identified on antigen or PCR testing, vancomycin should be added to the therapy. Table 9.2.3 Choice of antimicrobial in meningitis [16] After eTG complete [internet]. Melbourne: Therapeutic Guidelines Limited; 2013 July with permission. Table 9.2.4 Antibiotic doses in treating meningitis [17] After eTG complete [internet]. Melbourne: Therapeutic Guidelines Limited; 2013 July with permission. Steroids have been shown to improve the prognosis of bacterial meningitis in both adults and children. There is a reduction in complications, such as sensorineural deafness and short-term neurological deficits (in high-income countries). The most benefit appears to be derived with infections from H. influenzae and S. pneumoniae. No clear mortality benefit has been established. Steroids are usually administered as dexamethasone 0.15 mg/kg IV q 6 h (up to 10 mg), started before or with the first dose of antibiotics and continued for 4 days. The main adverse effect is gastrointestinal bleeding, which may be reduced by limiting treatment to 2 days [17]. All cases of bacterial meningitis require admission for IV antibiotics, as well as supportive therapy. They often require intensive therapy, especially if septic shock has supervened. Viral meningitis will usually require supportive therapy only, but this may require admission. Mild cases of viral or aseptic meningitis, with a clear diagnosis, can be safely sent home. Over the last 20 years, the mortality of bacterial meningitis has ranged from 6 to 20% and is higher in the very young or the very old. Meningitis in immunocompromised individuals carries a high mortality of up to 50%. Bacterial meningitis in children can lead to a number of long-term sequelae, such as sensorineural hearing loss, learning difficulties, motor problems, speech delay, hyperactivity, blindness, obstructive hydrocephalus and recurrent seizures. These sequelae are less common in adults. Prophylaxis should be offered in cases of H. influenzae type b, or Meningococcus infection to: ■ ciprofloxacin 500 mg orally as a single dose – preferred for females on oral contraceptives ■ ceftriaxone 250 mg (125 mg in children<12 years IM in 1% lignocaine – preferred in pregnant women ■ rifampicin 600 mg orally 12-hourly for 2 days (5 mg/kg in neonates<1 month, 10 mg/kg in children). ■ rifampicin 600 mg orally daily for 4 days (10 mg/kg in neonates<1 month, 20 mg/kg in children) ■ ceftriaxone 1 g IM daily for 2 days (50 mg/kg in children) Casual, neighbourhood or hospital contacts are not required to receive prophylaxis. Meningococcal vaccine should be considered in populations where cases are clustered. The vaccine is currently only available for serogroup C. Trevor Jackson and Varadarajulu Suresh
Infectious Disease Emergencies
9.1 Approach to undifferentiated fever in adults
Introduction
Approach
Step 1: identify the seriously ill patient who requires urgent intervention
Step 2: identify those with localized infections or easily diagnosable diseases
History
Illness
Previous state of health
Predisposing events
Epidemiology
Contact with similar diseases and known infectious diseases
Examination
 Heart: murmurs and pericardial rubs.
Heart: murmurs and pericardial rubs.
 Lungs: subtle crackles may be heard in pneumonic patients without respiratory symptoms.
Lungs: subtle crackles may be heard in pneumonic patients without respiratory symptoms.
 Marked muscle tenderness is a frequent sign of sepsis.
Marked muscle tenderness is a frequent sign of sepsis.
 Neck stiffness may be a clue to meningitis in a confused patient who cannot give a history.
Neck stiffness may be a clue to meningitis in a confused patient who cannot give a history.
 Any area that is covered, e.g. under plasters or bandages, for evidence of sepsis.
Any area that is covered, e.g. under plasters or bandages, for evidence of sepsis.
There are two caveats when assessing local symptoms and signs:
Step 3: look for the ‘at-risk’ patient
Clinical pointers: type of patient
Elderly patients
Alcoholic patients
Injecting drug users
Patients with diabetes mellitus
Febrile neutropaenic patients
Splenectomized patients
Other immunocompromised patients
Clinical pointers: exposure history
Overseas travellers or visitors
Contact with animals
Contact with meningococcal and Haemophilus meningitis
Clinical pointers: non-specific clinical features (Table 9.1.1)
Severe pain in muscles, neck or back
Impairment of conscious state
Vomiting
Severe headache in the presence of a normal CSF
Unexplained rash
Jaundice
Sore throat or dysphagia
Repeated rigors
Clinical pointers: evolution of illness (Table 9.1.2)
Step 4: a final caveat
Disease
Clues
Meningococcaemia
Myalgia, rash. May be none
Falciparum malaria
Travel history, blood film
Bacterial meningitis
Headache, change in conscious state, CSF findings
Post-splenectomy sepsis
Past history, abdominal scar
Toxic shock syndromes
Presence of shock and usually a rash
Infections in the febrile neutropaenic
Past history, blood film
Infective endocarditis
Past history, murmur, petechiae
Necrotizing soft-tissue infections
Pain, tenderness, erythema and swelling in skin/muscle, toxicity
Space-occupying infection of head and neck
Localizing symptoms and signs
Focal intracranial infections
Headache, change in conscious state, neurological signs, CT findings
Clinical investigations
Disposition
 patients with diabetes mellitus
patients with diabetes mellitus
 immunologically compromised patients
immunologically compromised patients
 overseas travellers or visitors
overseas travellers or visitors
Future research directions
9.2 Meningitis
Introduction
Definition
Classification
Aetiology
Bacterial
Viral
Bacterial
Other
Echovirus 6, 9,11, 30
Neonates (<3 months old):
Mycobacterium tuberculosis
Coxsackie viruses A9, A16, B1, B5, B6
Enterovirus 71 H Herpes simplex 1 & 2
Group B streptococcus (Streptococcus agalactiae)
Escherichia coli
Listeria monocytogenes
Cryptococcus neoformans (especially in immunocompromised) Aseptic
Cytomegalovirus Varicella zoster Epstein-Barr virus
Coagulase-negative Staphylococcus aureus
Pseudomonas aeruginosa
Children (<6 years old):
Haemophilus influenzae type b
Neisseria meningitidis
Streptococcus pneumoniae
Adults
Neisseria meningitidis (especially in young adults)
Streptococcus pneumoniae
Listeria monocytogenes (especially in adults over 50)
Klebsiella pneumoniae
Staphylococcus aureus
Escherichia coli (in the immunocompromised)
Aseptic
Viral
Fungal
Tuberculous
Spinal
Epidemiology
 Neonates: Table 9.2.1 shows the main causes of bacterial meningitis in neonates. There is an overall incidence of 0.17–0.32 cases per 1000 live births. There is 26% mortality, which is even higher in premature infants [3].
Neonates: Table 9.2.1 shows the main causes of bacterial meningitis in neonates. There is an overall incidence of 0.17–0.32 cases per 1000 live births. There is 26% mortality, which is even higher in premature infants [3].
 Children: until the introduction of Haemophilus influenzae type b (Hib) immunization in the early 1990 s, this organism was the major cause of bacterial meningitis in children under 5 years (until 1990, the incidence of childhood Hib meningitis was 26.3 per 100 000 [152 per 100 000 in Aboriginal children]) [4]. Between 1990 and 1996 there was a 94% reduction in the incidence of Hib disease. N. meningitidis and S. pneumoniae remain common causes of both meningitis and generalized sepsis [5]. The introduction of immunization programmes for some strains of both of these bacteria will reduce the incidence of meningitis caused by these organisms in the future, although it is important to understand that not all strains are covered by vaccines.
Children: until the introduction of Haemophilus influenzae type b (Hib) immunization in the early 1990 s, this organism was the major cause of bacterial meningitis in children under 5 years (until 1990, the incidence of childhood Hib meningitis was 26.3 per 100 000 [152 per 100 000 in Aboriginal children]) [4]. Between 1990 and 1996 there was a 94% reduction in the incidence of Hib disease. N. meningitidis and S. pneumoniae remain common causes of both meningitis and generalized sepsis [5]. The introduction of immunization programmes for some strains of both of these bacteria will reduce the incidence of meningitis caused by these organisms in the future, although it is important to understand that not all strains are covered by vaccines.
 Adults: N. meningitidis and S. pneumoniae are common causes in all age groups, with N. meningitides predominating in adults under 24 years. Listeria monocytogenes is more common in adults over 50 years. The overall incidence in adults is 3.8 per 100 000 population [6]. More unusual organisms occur in patients following neurosurgery or chronic illness, such as alcoholism, hepatic cirrhosis, chronic renal failure and connective tissue disease [7] (GNRs, coagulase-negative Staphylococcus aureus, Mycobacterium tuberculosis, Klebsiella pneumoniae).
Adults: N. meningitidis and S. pneumoniae are common causes in all age groups, with N. meningitides predominating in adults under 24 years. Listeria monocytogenes is more common in adults over 50 years. The overall incidence in adults is 3.8 per 100 000 population [6]. More unusual organisms occur in patients following neurosurgery or chronic illness, such as alcoholism, hepatic cirrhosis, chronic renal failure and connective tissue disease [7] (GNRs, coagulase-negative Staphylococcus aureus, Mycobacterium tuberculosis, Klebsiella pneumoniae).
 Patients with HIV/AIDS: Cryptococcus neoformans is relatively common, with an incidence of 5 per million of population or 10% of HIV-infected patients. Tuberculosis, Listeria, Klebsiella and syphilis are also causes of meningitis in this group, as well as viral causes of meningoencephalitis [8].
Patients with HIV/AIDS: Cryptococcus neoformans is relatively common, with an incidence of 5 per million of population or 10% of HIV-infected patients. Tuberculosis, Listeria, Klebsiella and syphilis are also causes of meningitis in this group, as well as viral causes of meningoencephalitis [8].
Pathogenesis
Presentation
History
Examination
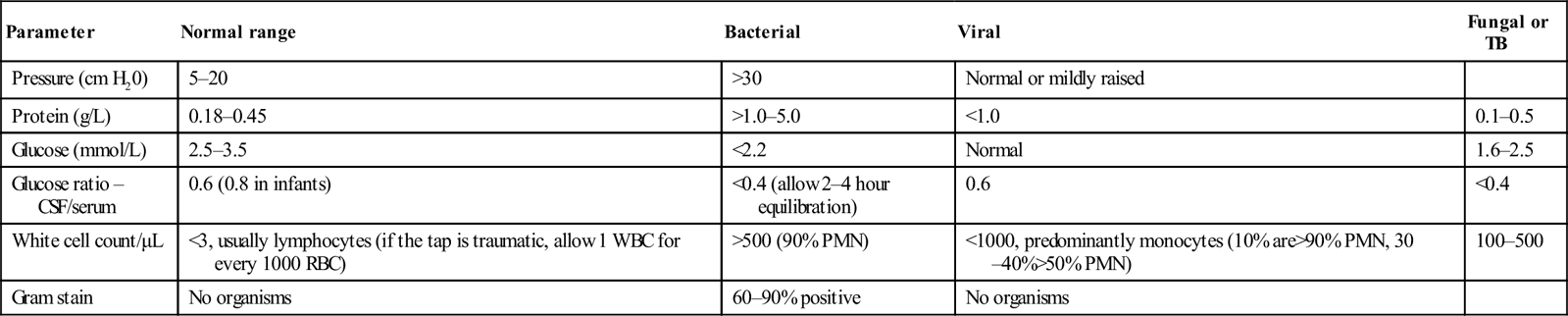
Investigations
Lumbar puncture
Indications
Precautions
Parameter
Normal range
Bacterial
Viral
Fungal or TB
Pressure (cm H20)
5–20
>30
Normal or mildly raised
Protein (g/L)
0.18–0.45
>1.0–5.0
<1.0
0.1–0.5
Glucose (mmol/L)
2.5–3.5
<2.2
Normal
1.6–2.5
Glucose ratio – CSF/serum
0.6 (0.8 in infants)
<0.4 (allow 2–4 hour equilibration)
0.6
<0.4
White cell count/μL
<3, usually lymphocytes (if the tap is traumatic, allow 1 WBC for every 1000 RBC)
>500 (90% PMN)
<1000, predominantly monocytes (10% are>90% PMN, 30–40%>50% PMN)
100–500
Gram stain
No organisms
60–90% positive
No organisms
CT scan
Microbiology
Skin lesion aspirate
Throat swab
Polymerase chain reaction
Serology
Antigenic studies
General investigations
Differential diagnosis
 Generalized viral infections, with meningism as a component.
Generalized viral infections, with meningism as a component.
 Brain abscess: this tends to produce focal signs due to local pressure at the site of the abscess.
Brain abscess: this tends to produce focal signs due to local pressure at the site of the abscess.
 Focal cerebral infections, such as those due to Toxoplasma gondii in HIV/AIDS patients.
Focal cerebral infections, such as those due to Toxoplasma gondii in HIV/AIDS patients.
 Severe pharyngitis with cervical lymphadenopathy causing neck stiffness.
Severe pharyngitis with cervical lymphadenopathy causing neck stiffness.
Management
General
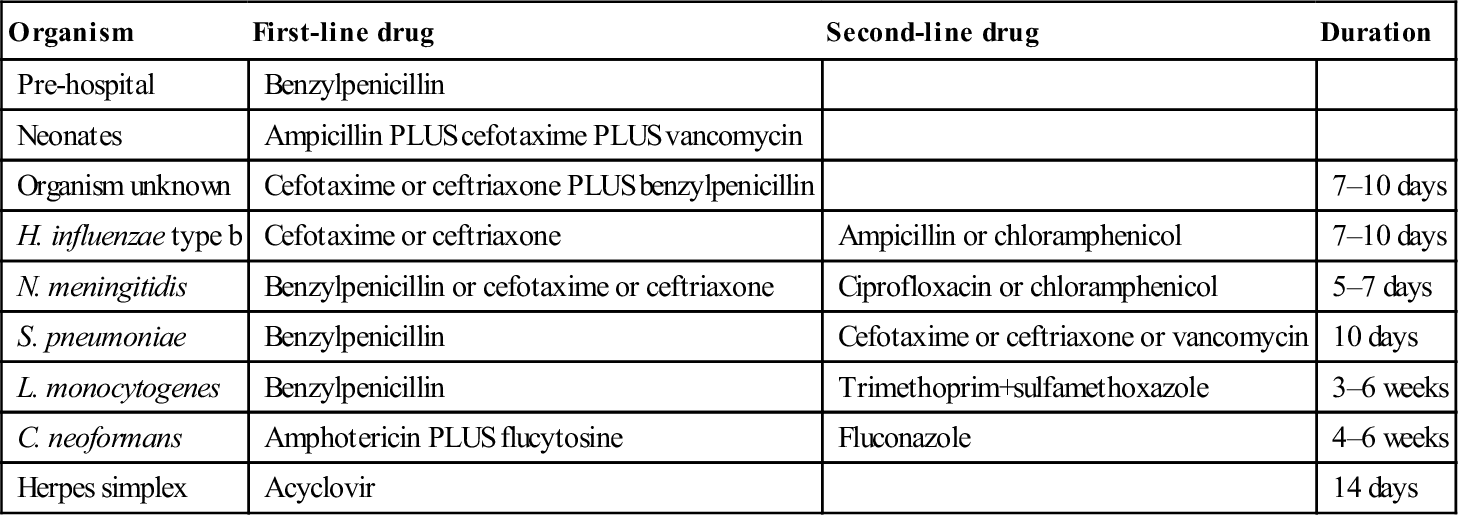
Antimicrobials
Organism
First-line drug
Second-line drug
Duration
Pre-hospital
Benzylpenicillin
Neonates
Ampicillin PLUS cefotaxime PLUS vancomycin
Organism unknown
Cefotaxime or ceftriaxone PLUS benzylpenicillin
7–10 days
H. influenzae type b
Cefotaxime or ceftriaxone
Ampicillin or chloramphenicol
7–10 days
N. meningitidis
Benzylpenicillin or cefotaxime or ceftriaxone
Ciprofloxacin or chloramphenicol
5–7 days
S. pneumoniae
Benzylpenicillin
Cefotaxime or ceftriaxone or vancomycin
10 days
L. monocytogenes
Benzylpenicillin
Trimethoprim+sulfamethoxazole
3–6 weeks
C. neoformans
Amphotericin PLUS flucytosine
Fluconazole
4–6 weeks
Herpes simplex
Acyclovir
14 days
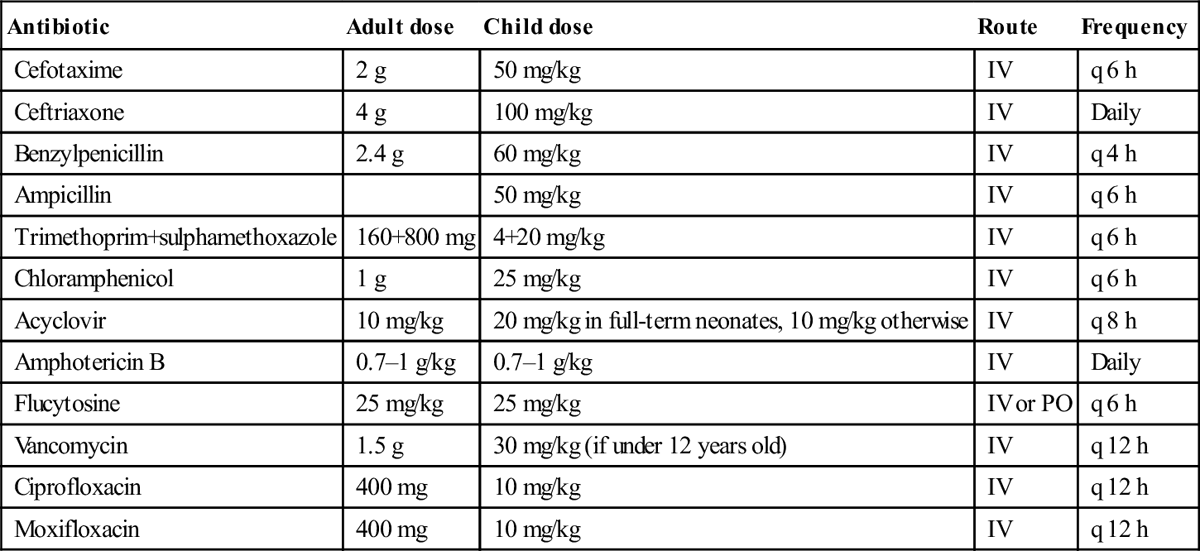
Antibiotic
Adult dose
Child dose
Route
Frequency
Cefotaxime
2 g
50 mg/kg
IV
q 6 h
Ceftriaxone
4 g
100 mg/kg
IV
Daily
Benzylpenicillin
2.4 g
60 mg/kg
IV
q 4 h
Ampicillin
50 mg/kg
IV
q 6 h
Trimethoprim+sulphamethoxazole
160+800 mg
4+20 mg/kg
IV
q 6 h
Chloramphenicol
1 g
25 mg/kg
IV
q 6 h
Acyclovir
10 mg/kg
20 mg/kg in full-term neonates, 10 mg/kg otherwise
IV
q 8 h
Amphotericin B
0.7–1 g/kg
0.7–1 g/kg
IV
Daily
Flucytosine
25 mg/kg
25 mg/kg
IV or PO
q 6 h
Vancomycin
1.5 g
30 mg/kg (if under 12 years old)
IV
q 12 h
Ciprofloxacin
400 mg
10 mg/kg
IV
q 12 h
Moxifloxacin
400 mg
10 mg/kg
IV
q 12 h
Steroids
Disposition
Prognosis
Prevention
 passengers adjacent to the index case on a trip of 8 hours’ or longer duration
passengers adjacent to the index case on a trip of 8 hours’ or longer duration
 healthcare workers who have given mouth-to-mouth resuscitation to an index case
healthcare workers who have given mouth-to-mouth resuscitation to an index case
9.3 Septic arthritis