Components
 Hypothalamus: hormones
Hypothalamus: hormones
 Corticotropin-releasing hormone (CRH): stimulates anterior pituitary adrenocorticotropic hormone (ACTH)
Corticotropin-releasing hormone (CRH): stimulates anterior pituitary adrenocorticotropic hormone (ACTH)
 Produced in response to a variety of signals (cold, fever, infection, trauma, emotional distress, burns, inflammatory agents, pain, hypotension, exercise, hemorrhage)
Produced in response to a variety of signals (cold, fever, infection, trauma, emotional distress, burns, inflammatory agents, pain, hypotension, exercise, hemorrhage)
 Thyrotropin-releasing hormone (TRH): stimulates anterior pituitary thyroid-stimulating hormone (TSH)
Thyrotropin-releasing hormone (TRH): stimulates anterior pituitary thyroid-stimulating hormone (TSH)
 Excess TRH inhibits dopamine, which stimulates prolactin release from anterior pituitary
Excess TRH inhibits dopamine, which stimulates prolactin release from anterior pituitary
 Antidiuretic hormone (ADH) or vasopressin: stored in the posterior pituitary
Antidiuretic hormone (ADH) or vasopressin: stored in the posterior pituitary
 Release stimulated by hypovolemia (carotid, atrial, venous baroreceptors), increased plasma oncotic pressure (hypothalamic osmoreceptors), and increased cholecystokinin
Release stimulated by hypovolemia (carotid, atrial, venous baroreceptors), increased plasma oncotic pressure (hypothalamic osmoreceptors), and increased cholecystokinin
 ADH acts on V1 receptors via the phosphatidyl inositol/calcium/protein kinase C pathway to mediate vasoconstriction, notably during hemorrhage or other hypovolemic hemodynamic instability
ADH acts on V1 receptors via the phosphatidyl inositol/calcium/protein kinase C pathway to mediate vasoconstriction, notably during hemorrhage or other hypovolemic hemodynamic instability
 ADH acts on V2 receptors (G protein coupled) via the cyclic AMP/protein kinase A pathway to create aquaporin-2 water channels in the apical membrane of distal renal tubule and collecting duct, allowing water reentry from filtrate back into bloodstream.
ADH acts on V2 receptors (G protein coupled) via the cyclic AMP/protein kinase A pathway to create aquaporin-2 water channels in the apical membrane of distal renal tubule and collecting duct, allowing water reentry from filtrate back into bloodstream.
 Gonadotropin-releasing hormone (GnRH): stimulates release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from anterior pituitary
Gonadotropin-releasing hormone (GnRH): stimulates release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from anterior pituitary
 Growth hormone-releasing hormone (GHRH): stimulates release of growth hormone (GH) from anterior pituitary
Growth hormone-releasing hormone (GHRH): stimulates release of growth hormone (GH) from anterior pituitary
 Somatostatin (SS): inhibits release of GH and TSH by anterior pituitary
Somatostatin (SS): inhibits release of GH and TSH by anterior pituitary
 Dopamine (DA): inhibits prolactin release from posterior pituitary
Dopamine (DA): inhibits prolactin release from posterior pituitary
 Oxytocin (OT): mediates uterine contraction and milk letdown reflex
Oxytocin (OT): mediates uterine contraction and milk letdown reflex
 Other hypothalamic functions include regulating temperature, hunger, thirst, sleep, and circadian cycles
Other hypothalamic functions include regulating temperature, hunger, thirst, sleep, and circadian cycles
 Pituitary
Pituitary
 Anterior pituitary
Anterior pituitary
 ACTH stimulates adrenal glucocorticoids
ACTH stimulates adrenal glucocorticoids
 TSH stimulates thyroid to release triiodothyronine (T3) and thyroxin (T4)
TSH stimulates thyroid to release triiodothyronine (T3) and thyroxin (T4)
 Beta endorphin
Beta endorphin
 Follicle-stimulating hormone (FSH)
Follicle-stimulating hormone (FSH)
 Luteinizing hormone (LH)
Luteinizing hormone (LH)
 Growth hormone (GH)
Growth hormone (GH)
 Prolactin
Prolactin
 Posterior pituitary
Posterior pituitary
 ADH/Vasopressin
ADH/Vasopressin
 Oxytocin
Oxytocin
 Autonomic nervous system: lateral hypothalamus
Autonomic nervous system: lateral hypothalamus
 Lateral hypothalamus projects to the lateral medulla (location of cells that drive the autonomic nervous system).
Lateral hypothalamus projects to the lateral medulla (location of cells that drive the autonomic nervous system).
 Parasympathetic vagal nuclei and cells project to the sympathetic system in the spinal cord.
Parasympathetic vagal nuclei and cells project to the sympathetic system in the spinal cord.
 Hypothalamus can control heart rate, digestion, sweating, and vasoconstriction.
Hypothalamus can control heart rate, digestion, sweating, and vasoconstriction.
 Primary critical care-related effector organs
Primary critical care-related effector organs
 Thyroid
Thyroid
 T4 or 3, 5, 3′, 5′-tetraiodothyronine: the major thyroid hormone secreted by thyroid follicular cells
T4 or 3, 5, 3′, 5′-tetraiodothyronine: the major thyroid hormone secreted by thyroid follicular cells
 T4 circulates as over 99% protein bound and is metabolized by thyroid peroxidase (TPO) to T3 in the tissues.
T4 circulates as over 99% protein bound and is metabolized by thyroid peroxidase (TPO) to T3 in the tissues.
 T3 is four times as potent as T4.
T3 is four times as potent as T4.
 Both T3 and T4 increase temperature and basal metabolic rate as well as affect protein synthesis, neuron maturation, and the metabolism of protein, carbohydrate, fat, and vitamins.
Both T3 and T4 increase temperature and basal metabolic rate as well as affect protein synthesis, neuron maturation, and the metabolism of protein, carbohydrate, fat, and vitamins.
 T4 and T3 also enhance sensitivity to catecholamines.
T4 and T3 also enhance sensitivity to catecholamines.
 T4 is converted to T3 in peripheral tissues by deiodinases.
T4 is converted to T3 in peripheral tissues by deiodinases.
 T3 is metabolically active.
T3 is metabolically active.
 Adrenal
Adrenal
 Cortex
Cortex
 Zonaglomerulosa (mineralocorticoids): aldosterone acts on the kidneys to provide:
Zonaglomerulosa (mineralocorticoids): aldosterone acts on the kidneys to provide:
 Active reabsorption of sodium
Active reabsorption of sodium
 Passive reabsorption of water
Passive reabsorption of water
 Secretion of potassium
Secretion of potassium
 Active secretion of protons via proton ATPases
Active secretion of protons via proton ATPases
 Increases in blood pressure and blood volume
Increases in blood pressure and blood volume
 Zonafasciculata (glucocorticoids)
Zonafasciculata (glucocorticoids)
 Immune effects: up-regulation of antiinflammatory proteins, down-regulation of proinflammatory proteins, role in development of T-lymphocytes
Immune effects: up-regulation of antiinflammatory proteins, down-regulation of proinflammatory proteins, role in development of T-lymphocytes
 Metabolic effects: stimulation of gluconeogenesis, mobilization of amino acids from extrahepatic tissues, inhibition of glucose uptake in muscle and adipose tissue, stimulation of fat breakdown in adipose tissue
Metabolic effects: stimulation of gluconeogenesis, mobilization of amino acids from extrahepatic tissues, inhibition of glucose uptake in muscle and adipose tissue, stimulation of fat breakdown in adipose tissue
 Zonareticularis: androgens
Zonareticularis: androgens
 Medulla
Medulla
 Epinephrine (80%)
Epinephrine (80%)
 Increases heart rate
Increases heart rate
 Constricts blood vessels
Constricts blood vessels
 Dilates air passages
Dilates air passages
 Participates in the “fight-or-flight” response of the sympathetic nervous system
Participates in the “fight-or-flight” response of the sympathetic nervous system
 Norepinephrine (20%)
Norepinephrine (20%)
 Increases heart rate
Increases heart rate
 Triggering the release of glucose from energy stores
Triggering the release of glucose from energy stores
 Increases blood flow to skeletal muscle
Increases blood flow to skeletal muscle
 Suppresses neuroinflammation
Suppresses neuroinflammation
 Negative feedback: exerted by secreted cortisol at the level of both the hypothalamus and the pituitary to reduce secretion of both CRH and ACTH
Negative feedback: exerted by secreted cortisol at the level of both the hypothalamus and the pituitary to reduce secretion of both CRH and ACTH
 Cortisol normally is secreted in a diurnal pattern, with a maximal circulatory level early in the morning, followed by a steady decrease throughout the day.
Cortisol normally is secreted in a diurnal pattern, with a maximal circulatory level early in the morning, followed by a steady decrease throughout the day.
SUGGESTED READINGS
Asensio JA, Trunkey DD. Current Therapy of Trauma and Surgical Critical Care. Philadelphia, PA: Mosby; 2008.
Cameron J, Cameron A. Current Surgical Therapy. 10th ed. Philadelphia, PA: Elsevier Saunders; 2011.
Marino PL. The ICU Book. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2007.
8.2
Diabetes Mellitus
Tariq A. Kelker and Rebecca Aslakson
Hyperglycemia
Most common metabolic disturbance seen in ICU.
 Differential Diagnosis
Differential Diagnosis
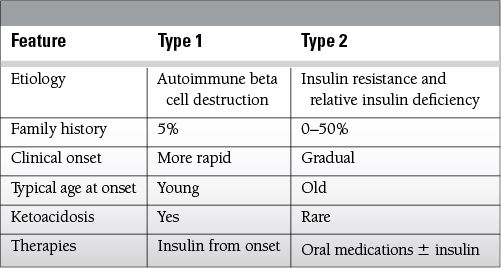
 Previously diagnosed or undiagnosed diabetes mellitus
Previously diagnosed or undiagnosed diabetes mellitus
 Stress hyperglycemia: factors released during acute illness and stress, including cytokines, growth hormone, glucagon, catecholamines, and cortisol
Stress hyperglycemia: factors released during acute illness and stress, including cytokines, growth hormone, glucagon, catecholamines, and cortisol
 Iatrogenic/exogenous: TPN, exogenous glucocorticoids
Iatrogenic/exogenous: TPN, exogenous glucocorticoids
 Subcutaneous or basal insulin is the treatment of choice for total parenteral nutrition (TPN) or glucocorticoids-related hyperglycemia.
Subcutaneous or basal insulin is the treatment of choice for total parenteral nutrition (TPN) or glucocorticoids-related hyperglycemia.
 IV insulin infusion may become necessary if subcutaneous or basal insulin doses cannot keep patient glucose <180 mg/dL threshold.
IV insulin infusion may become necessary if subcutaneous or basal insulin doses cannot keep patient glucose <180 mg/dL threshold.
 Sequelae
Sequelae
 Associated with worse outcome in medical and surgical illnesses
Associated with worse outcome in medical and surgical illnesses
 Elevated blood glucose may impair immune function by decreasing neutrophil adherence, chemotaxis, phagocytosis, complement fixation, and collagen deposition.
Elevated blood glucose may impair immune function by decreasing neutrophil adherence, chemotaxis, phagocytosis, complement fixation, and collagen deposition.
 It also impairs granulocyte adherence, bactericidal activity, and microbial killing (oxidative burst) and contributes to glycosylation of immunoglobulins.
It also impairs granulocyte adherence, bactericidal activity, and microbial killing (oxidative burst) and contributes to glycosylation of immunoglobulins.
 Diagnosis
Diagnosis
 Preexisting diagnosis of diabetes
Preexisting diagnosis of diabetes
 Two sequential blood glucose measurements of 150 mg/dL or greater
Two sequential blood glucose measurements of 150 mg/dL or greater
 Management and Treatment
Management and Treatment
 ICU-related: elevated blood glucose > 150 mg/dL on sequential measurements
ICU-related: elevated blood glucose > 150 mg/dL on sequential measurements
 Intensive sliding scale (blood glucose checked every 4 hours)
Intensive sliding scale (blood glucose checked every 4 hours)
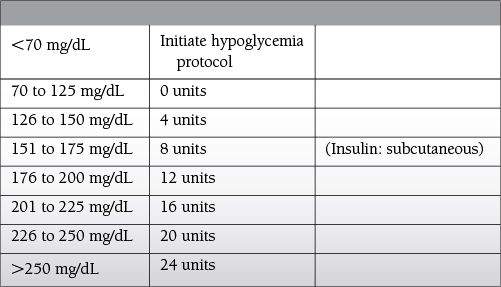
 Elevated blood glucose > 200 mg/dL on sequential measurements
Elevated blood glucose > 200 mg/dL on sequential measurements
 Continuous insulin infusion should be considered
Continuous insulin infusion should be considered
 Consider dosing of long-acting insulin, such as glargine (Lantus) or (NPH)
Consider dosing of long-acting insulin, such as glargine (Lantus) or (NPH)
 Determine amount of regular insulin (from infusion or sliding scale) used in the previous 24-hour period
Determine amount of regular insulin (from infusion or sliding scale) used in the previous 24-hour period
 Administer 1/2 of above amount as long-acting insulin every 24 hours
Administer 1/2 of above amount as long-acting insulin every 24 hours
Hypoglycemia
Major risk factor of insulin infusion protocols or any program of tight glycemic control.
 Differential diagnosis
Differential diagnosis
 Common complication of insulin treatment!
Common complication of insulin treatment!
 Undetected or unexpected interruption of calorie intake
Undetected or unexpected interruption of calorie intake
 Prior administration of long-acting insulin secretagogues such as glyburide coupled with renal insufficiency can result in prolonged (>72 hours) hypoglycemia
Prior administration of long-acting insulin secretagogues such as glyburide coupled with renal insufficiency can result in prolonged (>72 hours) hypoglycemia
 Fulminant hepatic failure or severe end-stage liver disease
Fulminant hepatic failure or severe end-stage liver disease
 Rare tumors, such as insulinomas, leading to Whipple triad of hypoglycemia (symptoms consistent with hypoglycemia, a low blood sugar, resolution of symptoms after administration of glucose)
Rare tumors, such as insulinomas, leading to Whipple triad of hypoglycemia (symptoms consistent with hypoglycemia, a low blood sugar, resolution of symptoms after administration of glucose)
 Symptoms
Symptoms
 Primary symptoms: diaphoresis, coldness, clamminess, irritability, confusion, combativeness, seizure, coma
Primary symptoms: diaphoresis, coldness, clamminess, irritability, confusion, combativeness, seizure, coma
 Physiologic responses to hypoglycemia: tachycardia, hypertension, mydriasis
Physiologic responses to hypoglycemia: tachycardia, hypertension, mydriasis
 Diagnosis
Diagnosis
 Blood glucose <50 mg/dL: in critical care setting frequent glucose testing is recommended.
Blood glucose <50 mg/dL: in critical care setting frequent glucose testing is recommended.
 Management and treatment
Management and treatment
 IV bolus of 50% dextrose solution (D50): 50 mL provides 25 g of glucose and raises blood glucose, on average, by 125 mg/dL
IV bolus of 50% dextrose solution (D50): 50 mL provides 25 g of glucose and raises blood glucose, on average, by 125 mg/dL
To avoid overcorrection of hypoglycemia, the amount of D50 solution necessary to bring glucose levels back to 100 mg/dL can be calculated from the formula:
(100 − current BG) × 0.4 = mL of D50% solution as IV
Diabetic Ketoacidosis
 Key pathophysiology
Key pathophysiology
 Develops in type 1 and some insulin-dependent type 2 diabetic patients
Develops in type 1 and some insulin-dependent type 2 diabetic patients
 Lack of insulin prevents glucose release from cells and lipids become the primary energy source.
Lack of insulin prevents glucose release from cells and lipids become the primary energy source.
 Consequent lipolysis directly produces ketoacids; lack of insulin also increases catecholamine release that further accelerates lipolysis.
Consequent lipolysis directly produces ketoacids; lack of insulin also increases catecholamine release that further accelerates lipolysis.
 Differential diagnosis
Differential diagnosis
 Surgical stress, infection, myocardial infarction, trauma, and failure to supply insulin
Surgical stress, infection, myocardial infarction, trauma, and failure to supply insulin
 Sequelae
Sequelae
 Severe complications associated with diabetic ketoacidosis (DKA) can be dire, and include cerebral edema, adult respiratory distress syndrome (ARDS), and severe life-threatening acidosis, volume depletion, and electrolyte abnormalities.
Severe complications associated with diabetic ketoacidosis (DKA) can be dire, and include cerebral edema, adult respiratory distress syndrome (ARDS), and severe life-threatening acidosis, volume depletion, and electrolyte abnormalities.
 Symptoms
Symptoms
 Usually caused by severe acidosis: hyperventilation, tachycardia, hypotension with postural features, nausea, vomiting, and abdominal pain that can mimic an acute abdomen
Usually caused by severe acidosis: hyperventilation, tachycardia, hypotension with postural features, nausea, vomiting, and abdominal pain that can mimic an acute abdomen
 Diagnosis
Diagnosis
 The diagnostic hallmarks of this syndrome include severe anion gap metabolic acidosis, hyperglycemia, hyperosmolality, serum and urine ketones, glycosuria, and polyuria leading to marked hypovolemia, culminating in prerenal azotemia.
The diagnostic hallmarks of this syndrome include severe anion gap metabolic acidosis, hyperglycemia, hyperosmolality, serum and urine ketones, glycosuria, and polyuria leading to marked hypovolemia, culminating in prerenal azotemia.
 Glucose levels are generally >300 mg/dL but can be lower in underfed, malnourished patient.
Glucose levels are generally >300 mg/dL but can be lower in underfed, malnourished patient.
 Serum pH is >7.2, unless it is a mixed acid-base disturbance (as can often occur with severe vomiting).
Serum pH is >7.2, unless it is a mixed acid-base disturbance (as can often occur with severe vomiting).
 Elevated concentrations of serum acetone, betahydroxybutyrate, as well as urine acetone
Elevated concentrations of serum acetone, betahydroxybutyrate, as well as urine acetone
 Serum osmolality is usually >300 mOsm/L.
Serum osmolality is usually >300 mOsm/L.
 Total body stores of potassium are depleted.
Total body stores of potassium are depleted.
 However, initial serum potassium often high due to renal impairment and acidosis with shift in potassium to extra-cellular space
However, initial serum potassium often high due to renal impairment and acidosis with shift in potassium to extra-cellular space
 Blood urea nitrogen (BUN) and creatinine usually elevated with acute renal failure due to prerenal azotemia in the setting of severe dehydration
Blood urea nitrogen (BUN) and creatinine usually elevated with acute renal failure due to prerenal azotemia in the setting of severe dehydration
 Initial apparent hyponatremia, however, when corrected for hyperglycemia, actual hypernatremia often present
Initial apparent hyponatremia, however, when corrected for hyperglycemia, actual hypernatremia often present
 Frequent hypophosphotemia and hypomagnesemia on presentation
Frequent hypophosphotemia and hypomagnesemia on presentation
 Management and treatment
Management and treatment
 Patients are severely dehydrated with potential life-threatening electrolyte abnormalities.
Patients are severely dehydrated with potential life-threatening electrolyte abnormalities.
 Hemodynamic monitoring and adequate IV access is essential.
Hemodynamic monitoring and adequate IV access is essential.
 Fluid resuscitation: 0.9% sodium chloride ~1 L/h (0.45% saline can be used in patients with severe hypernatremia (>155 mEq/L)).
Fluid resuscitation: 0.9% sodium chloride ~1 L/h (0.45% saline can be used in patients with severe hypernatremia (>155 mEq/L)).
 Regular (short-acting insulin): loading dose: 0.1 to 0.15 U/kg IV bolus; maintenance dose: 0.1 U/kg/h IV infusion, typically 5 to 7 U/h
Regular (short-acting insulin): loading dose: 0.1 to 0.15 U/kg IV bolus; maintenance dose: 0.1 U/kg/h IV infusion, typically 5 to 7 U/h
 Replete potassium: 20 to 40 mEq/L of KCl can be added to each liter of resuscitation fluid.
Replete potassium: 20 to 40 mEq/L of KCl can be added to each liter of resuscitation fluid.
 Frequent monitoring of glucose levels per an insulin-infusion protocol should be done and chemistry profiles taken every 4 to 6 hours initially.
Frequent monitoring of glucose levels per an insulin-infusion protocol should be done and chemistry profiles taken every 4 to 6 hours initially.
 Begin 5% dextrose when glucose reaches ~200 mg/dL.
Begin 5% dextrose when glucose reaches ~200 mg/dL.
 Reserve sodium bicarbonate for pH <7.0.
Reserve sodium bicarbonate for pH <7.0.
 Consider hypertonic saline 2% to 3%, hyperventilation, and slowing the IV fluid replacement if neurologic deterioration occurs due to cerebral edema.
Consider hypertonic saline 2% to 3%, hyperventilation, and slowing the IV fluid replacement if neurologic deterioration occurs due to cerebral edema.
 Identify and treat any DKA-precipitating causes such as infection.
Identify and treat any DKA-precipitating causes such as infection.
 Once patient is stabilized, transition from insulin infusion to subcutaneous administration.
Once patient is stabilized, transition from insulin infusion to subcutaneous administration.
Hyperosmolar Non-Ketotic
 Key pathophysiology
Key pathophysiology
 Hyperglycemia syndrome: typically in type 2 diabetics, relative insulin deficiency without an absolute absence of insulin, no ketoacid production
Hyperglycemia syndrome: typically in type 2 diabetics, relative insulin deficiency without an absolute absence of insulin, no ketoacid production
 Differential diagnosis
Differential diagnosis
 Differential diagnosis is similar to DKA and includes surgery, renal insults, myocardial infarction, stroke, and infection; iatrogenic factors include drugs (such as diuretics and/or corticosteroids) and therapies (such as hyperalimentation).
Differential diagnosis is similar to DKA and includes surgery, renal insults, myocardial infarction, stroke, and infection; iatrogenic factors include drugs (such as diuretics and/or corticosteroids) and therapies (such as hyperalimentation).
 Patients are often older, with underlying impaired renal function.
Patients are often older, with underlying impaired renal function.
 They are less able to excrete glucose through the kidneys.
They are less able to excrete glucose through the kidneys.
 Serum glucose levels can exceed 600 to 800 g/dL with consequent progressive hyperosmolality
Serum glucose levels can exceed 600 to 800 g/dL with consequent progressive hyperosmolality
 Sequelae
Sequelae
 Cerebral edema
Cerebral edema
 Symptoms
Symptoms
 Tachycardia, hypotension, decreased mentation, seizures
Tachycardia, hypotension, decreased mentation, seizures
 Patients are often profoundly volume depleted and dehydrated.
Patients are often profoundly volume depleted and dehydrated.
 Confusion and generalized or focal neurologic deficits are present.
Confusion and generalized or focal neurologic deficits are present.
 Fluid deficits may exceed 10 L and renal impairment with creatinine levels > 3 mg/dL are common
Fluid deficits may exceed 10 L and renal impairment with creatinine levels > 3 mg/dL are common
 Serum osmolality often exceeds 330 mOsm/L
Serum osmolality often exceeds 330 mOsm/L
 Potassium deficits exist but are not as severe as in DKA.
Potassium deficits exist but are not as severe as in DKA.
 Diagnosis
Diagnosis
 Hyperglycemia with glucose > 600
Hyperglycemia with glucose > 600
 Hyperosmolality (>330 mOsmol/kg)
Hyperosmolality (>330 mOsmol/kg)
 No serum ketones
No serum ketones
 Management and treatment
Management and treatment
 Aggressive volume resuscitation with isotonic saline (to correct hemodynamic instability) followed by 0.45% saline to replace free water losses and correct hyperosmolality
Aggressive volume resuscitation with isotonic saline (to correct hemodynamic instability) followed by 0.45% saline to replace free water losses and correct hyperosmolality
 Insulin administration to move glucose into cells and to assist in lowering osmolality
Insulin administration to move glucose into cells and to assist in lowering osmolality
 An IV insulin protocol is useful, but lower dosing may be sufficient
An IV insulin protocol is useful, but lower dosing may be sufficient
 As compared to DKA, hyperosmolar non-ketotic (HONK) patients are often more sensitive to insulin and fluids
As compared to DKA, hyperosmolar non-ketotic (HONK) patients are often more sensitive to insulin and fluids
 Correction of the hyperosmolality should proceed in a gradual fashion over 2 to 24 hours to avoid the risk of cerebral edema
Correction of the hyperosmolality should proceed in a gradual fashion over 2 to 24 hours to avoid the risk of cerebral edema
SUGGESTED READINGS
Carlotti AP, Bohn D, Kamel KS, et al. Minimizing the risk of developing cerebral edema during therapy for diabetic ketoacidosis. Crit Care Med. 2007;35:1450.
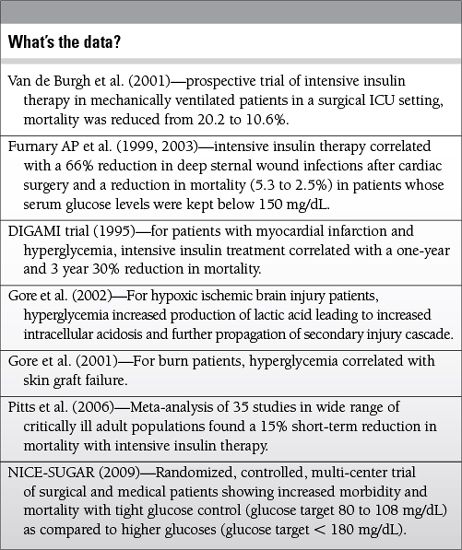
Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2003;125:1007-1021.
Furnary AP, Zerr KJ, Grunkemeier GL, et al. Continuous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352-362.
Gandhi GY, Nuthall GA, Abel MD, et al. Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients. Mayo Clin Proc. 2005;80:862-866.
Goldberg PA, Siegel MD, Sherman RS, et al. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diab Care. 2004;27:461-467.
Gore DC, Chinkes D, Heggers JP, et al. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51: 540-544.
Gore DC, Chinkes DL, Hart DW. Hyperglycemia exacerbates muscle protein catabolism in burn injured patients. Crit Care Med. 2002;30:2438-2442.
Kitabachi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diab Care. 2001;24:131-153.
Magee MF, Bhatt BA. Management of decompensated diabetes. Diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome. Crit Care Clin. 2001;17:75-106.
Malberg K, Ryden L, Efendic S, et al. Randomized trail of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI); effects on mortality at one year. J Am CollCardiol. 1995;26:57-65.
Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized controlled trials. Arch Int Med. 2006;164:2005-2011.
The NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283-1297.
Van den Bergh G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367.
8.3
Adrenal Gland
Ranjit Deshpande and Rebecca Aslakson
Adrenal Insufficiency
Differential Diagnosis
 Primary/absolute
Primary/absolute
 Rare, incidence < 0.015% in general population
Rare, incidence < 0.015% in general population
 Multiple potential causes including isolated autoimmune adrenalitis, autoimmune polyglandular syndrome (APS), infection, adrenal hemorrhage, infiltration with tumor, congenital adrenal hyperplasia, adrenoleukodystrophy, and/or secondary to drugs (such as etomidate, ketoconazole, or RU486)
Multiple potential causes including isolated autoimmune adrenalitis, autoimmune polyglandular syndrome (APS), infection, adrenal hemorrhage, infiltration with tumor, congenital adrenal hyperplasia, adrenoleukodystrophy, and/or secondary to drugs (such as etomidate, ketoconazole, or RU486)
 Associated symptoms typically include hypoparathyroidism, chronic mucocutaneous candidiasis, hyperthyroidism, premature ovarian failure, vitiligo, DM type 1, pernicious anemia
Associated symptoms typically include hypoparathyroidism, chronic mucocutaneous candidiasis, hyperthyroidism, premature ovarian failure, vitiligo, DM type 1, pernicious anemia
 Secondary adrenal insufficiency (AI)
Secondary adrenal insufficiency (AI)
 Dysfunction of the HPA axis and only glucocorticoid deficiency present
Dysfunction of the HPA axis and only glucocorticoid deficiency present
 Multiple potential causes include pituitary tumors (e.g. craniopharyngioma, meningioma, ependymoma, or metastases), irradiation to the pituitary, infiltration (e.g. TB or sarcoid), Wegener autoimmune hypophysitis, chronic glucocorticoid use, proopiomelanocortin deficiency, combined pituitary hormone deficiency, congenital absent ACTH.
Multiple potential causes include pituitary tumors (e.g. craniopharyngioma, meningioma, ependymoma, or metastases), irradiation to the pituitary, infiltration (e.g. TB or sarcoid), Wegener autoimmune hypophysitis, chronic glucocorticoid use, proopiomelanocortin deficiency, combined pituitary hormone deficiency, congenital absent ACTH.
 Acute AI
Acute AI
 Most typically in patients taking exogenous steroids who do not receive further steroid supplementation during stress (such as perioperative)
Most typically in patients taking exogenous steroids who do not receive further steroid supplementation during stress (such as perioperative)
 Loss of or impaired glucocorticoid and mineralocorticoid secretion
Loss of or impaired glucocorticoid and mineralocorticoid secretion
 Postural hypotension may progress to hypovolemic shock.
Postural hypotension may progress to hypovolemic shock.
 Patients may present with an acute abdomen or with decreased responsiveness, progressing to stupor and coma.
Patients may present with an acute abdomen or with decreased responsiveness, progressing to stupor and coma.
 Ongoing illness or stress (e.g., surgical) can precipitate an adrenal crisis with resultant profound and persistent hypotension.
Ongoing illness or stress (e.g., surgical) can precipitate an adrenal crisis with resultant profound and persistent hypotension.
 Relative AI
Relative AI
 Controversial concept that severely ill patients (typically in ICUs with sepsis) have a suppressed adrenal axis
Controversial concept that severely ill patients (typically in ICUs with sepsis) have a suppressed adrenal axis
 Synonym is “vasopressor-dependent septic shock”
Synonym is “vasopressor-dependent septic shock”
Signs and Symptoms
 Glucocorticoid deficiency
Glucocorticoid deficiency
 Fatigue, weight loss, anorexia, myalgias, fever, anemia, eosinophilia, hypoglycemia, hyponatremia, postural hypotension
Fatigue, weight loss, anorexia, myalgias, fever, anemia, eosinophilia, hypoglycemia, hyponatremia, postural hypotension
 Mineralocorticoid deficiency (primary AI)
Mineralocorticoid deficiency (primary AI)
 Abdominal pain, nausea, vomiting, dizziness, postural hypotension, hyponatremia, hyperkalemia, skin changes (hyperpigmentation in primary AI, alabaster pale skin in secondary AI)
Abdominal pain, nausea, vomiting, dizziness, postural hypotension, hyponatremia, hyperkalemia, skin changes (hyperpigmentation in primary AI, alabaster pale skin in secondary AI)
 Adrenal androgen deficiency
Adrenal androgen deficiency
 Fatigue, pruritus, decreased libido, lack of axillary hair
Fatigue, pruritus, decreased libido, lack of axillary hair
Diagnosis
 Random cortisol < 10 μcg/dL
Random cortisol < 10 μcg/dL
 Cosyntropin stimulation test
Cosyntropin stimulation test
 250 μcg IV ACTH administered with cortisol levels measured at baseline, 30, and 60 minutes
250 μcg IV ACTH administered with cortisol levels measured at baseline, 30, and 60 minutes
 Negative test—increase > 9 μcg/dL or total >18 to 20 ug/dL
Negative test—increase > 9 μcg/dL or total >18 to 20 ug/dL
 If test positive, primary versus secondary AI determined by ACTH
If test positive, primary versus secondary AI determined by ACTH
 Primary AI—ACTH increased
Primary AI—ACTH increased
 Secondary AI—ACTH decreased
Secondary AI—ACTH decreased
 Controversy exists regarding the reliability of the cosyntropin test to diagnose relative AI
Controversy exists regarding the reliability of the cosyntropin test to diagnose relative AI
Management and Treatment
 Do not delay treatment to perform diagnostic tests.
Do not delay treatment to perform diagnostic tests.
 Primary AI: hormone replacement is the mainstay
Primary AI: hormone replacement is the mainstay
 Hydrocortisone 20 to 30 mg/d with meals
Hydrocortisone 20 to 30 mg/d with meals
 Two-thirds of the dose in the morning and remaining late in the afternoon
Two-thirds of the dose in the morning and remaining late in the afternoon
 Fludrocortisone 0.05 to 0.1 mg daily PO
Fludrocortisone 0.05 to 0.1 mg daily PO
 Patient should also maintain good sodium intake 3 to 4 g/d.
Patient should also maintain good sodium intake 3 to 4 g/d.
 Females might need dihydroepiandrosterone (DHEA) orally.
Females might need dihydroepiandrosterone (DHEA) orally.
 Medical identification bracelets and patient education
Medical identification bracelets and patient education
 Treatment is monitored by BP and electrolyte changes, and sometimes through plasma renin concentrations.
Treatment is monitored by BP and electrolyte changes, and sometimes through plasma renin concentrations.
 Secondary AI
Secondary AI
 Glucocorticoid therapy is primary treatment, with doses similar to those for primary AI.
Glucocorticoid therapy is primary treatment, with doses similar to those for primary AI.
 Acute AI
Acute AI
 Immediate rehydration-saline infusion at initial rates of up to 1 L/h with continuous cardiac monitoring
Immediate rehydration-saline infusion at initial rates of up to 1 L/h with continuous cardiac monitoring
 Hydrocortisone 100 mg IV bolus, then 100 to 200 mg daily by either continuous infusion or IV/IM bolus
Hydrocortisone 100 mg IV bolus, then 100 to 200 mg daily by either continuous infusion or IV/IM bolus
 Fludrocortisone administration if daily hydrocortisone <50 mg
Fludrocortisone administration if daily hydrocortisone <50 mg
 Relative AI: hotly debated whether or not to treat with steroids
Relative AI: hotly debated whether or not to treat with steroids
 A standard approach is to administer dexamethasone 6 mg IV every 6 hours and fludrocortisone 50 μcg PO daily while awaiting cosyntropin stimulation test results.
A standard approach is to administer dexamethasone 6 mg IV every 6 hours and fludrocortisone 50 μcg PO daily while awaiting cosyntropin stimulation test results.
 Alternative treatment
Alternative treatment
 Hydrocortisone 50 mg IV every 6 hours
Hydrocortisone 50 mg IV every 6 hours
 Hydrocortisone infusion approximately 10 mg/h for 7 days
Hydrocortisone infusion approximately 10 mg/h for 7 days
 Methylprednisolone 1 mg/kg once daily for approximately 14 days
Methylprednisolone 1 mg/kg once daily for approximately 14 days

Pheochromocytoma—Tumor of Sympathoadrenalchromaffin Cell Tumors
Differential Diagnosis
 Primary, approximately 75% of cases
Primary, approximately 75% of cases
 Part of syndrome, approximately 25% of cases
Part of syndrome, approximately 25% of cases
 Multiple endocrine neoplasia (MEN) 2A (medullary thyroid carcinoma, pheochromocytoma, hyperparathyroidism)
Multiple endocrine neoplasia (MEN) 2A (medullary thyroid carcinoma, pheochromocytoma, hyperparathyroidism)
 MEN 2B (medullary thyroid carcinoma, pheochromocytoma, mucosal neuromas, marfanoid habitus, developmental disorder)
MEN 2B (medullary thyroid carcinoma, pheochromocytoma, mucosal neuromas, marfanoid habitus, developmental disorder)
 Hippel-Lindau syndrome (retinal and cerebellar hemangioblastomas, clear cell renal carcinomas, pancreatic islet cell tumors, endolymphatic sac tumors, pheochromocytoma, pancreatic, and/or renal cysts)
Hippel-Lindau syndrome (retinal and cerebellar hemangioblastomas, clear cell renal carcinomas, pancreatic islet cell tumors, endolymphatic sac tumors, pheochromocytoma, pancreatic, and/or renal cysts)
 Neurofibromatosis type 1 (multiple neurofibromas, café au lait spots, axillary freckling, Lisch nodules, pheochromocytoma)
Neurofibromatosis type 1 (multiple neurofibromas, café au lait spots, axillary freckling, Lisch nodules, pheochromocytoma)
“Rule of 10s”
10% bilateral, 10% malignant, and 10% extraadrenal—percentages are higher in syndrome-associated pheochromocytomas.
Symptoms
 Hypertension and triad of episodic palpitations, headache, and sweating
Hypertension and triad of episodic palpitations, headache, and sweating
 Typical ICU presentation: hypertensive crisis after angiography or during the perioperative period
Typical ICU presentation: hypertensive crisis after angiography or during the perioperative period
Diagnosis
 Laboratory: plasma and urine catecholamines, urine metanephrines, clonidine suppression test
Laboratory: plasma and urine catecholamines, urine metanephrines, clonidine suppression test
 Imaging: CT (risk of precipitating hypertensive crisis) or MRI; 123-I-metaiodobenzylguanidine (MIBG) scan, octreotide scan, selective venous sampling, positron emission scan
Imaging: CT (risk of precipitating hypertensive crisis) or MRI; 123-I-metaiodobenzylguanidine (MIBG) scan, octreotide scan, selective venous sampling, positron emission scan
Management and Treatment
 Medical management of pheochromocytoma: alpha blockade followed by beta blockade
Medical management of pheochromocytoma: alpha blockade followed by beta blockade