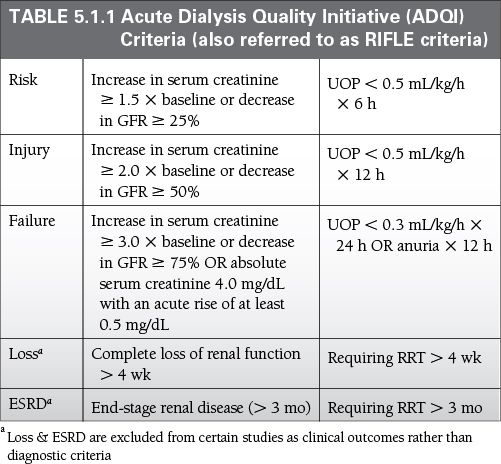
Increase in serum creatinine ≥0.3 mg/dL within 48 hours
 Increase in serum creatinine ≥1.5 × baseline, which is known or presumed to have occurred within the prior 7 days
Increase in serum creatinine ≥1.5 × baseline, which is known or presumed to have occurred within the prior 7 days
 UOP <0.5 mL/kg/h for 6 hours (Table 5.1.1)
UOP <0.5 mL/kg/h for 6 hours (Table 5.1.1)
 ADQI/RIFLE and AKIN Criteria have been shown to be diagnostically similar in ICU patients.
ADQI/RIFLE and AKIN Criteria have been shown to be diagnostically similar in ICU patients.
 UOP may better predict mortality in the ICU than serum creatinine in AKI.
UOP may better predict mortality in the ICU than serum creatinine in AKI.
Epidemiology
 Affects 5% to 25% of patients in the ICU, largest study showed 5.7%
Affects 5% to 25% of patients in the ICU, largest study showed 5.7%
 Median age 67
Median age 67
 Sepsis is the most common contributing condition followed by major surgery, cardiogenic shock, hypovolemia, and medications.
Sepsis is the most common contributing condition followed by major surgery, cardiogenic shock, hypovolemia, and medications.
Key Pathophysiology
 Renal Blood Flow (RBF) – at baseline ~1.1 L/min or ~20% of cardiac output
Renal Blood Flow (RBF) – at baseline ~1.1 L/min or ~20% of cardiac output
 Autoregulation maintains RBF (and therefore GFR) remarkably stable at SBP 90 to 200
Autoregulation maintains RBF (and therefore GFR) remarkably stable at SBP 90 to 200
 Myogenic = ↑ perfusion pressure causes ↑ stretch of smooth muscle in afferent arterioles which causes smooth muscle contraction (calcium-mediated), which causes ↑ resistance and ↓ RBF.
Myogenic = ↑ perfusion pressure causes ↑ stretch of smooth muscle in afferent arterioles which causes smooth muscle contraction (calcium-mediated), which causes ↑ resistance and ↓ RBF.
 Tubuloglomerular Feedback = ↑ RBF causes ↑ delivery of sodium to the macula densa which causes the release of local vasoconstrictors (likely adenosine) which causes ↑ resistance and ↓ RBF.
Tubuloglomerular Feedback = ↑ RBF causes ↑ delivery of sodium to the macula densa which causes the release of local vasoconstrictors (likely adenosine) which causes ↑ resistance and ↓ RBF.
 Sympathetic Nervous System = SNS activation (hemorrhage, surgery, etc.) causes release of norepinephrine which causes renal vasoconstriction and mesangial cell contraction which ↓ RBF > ↓ GFR.
Sympathetic Nervous System = SNS activation (hemorrhage, surgery, etc.) causes release of norepinephrine which causes renal vasoconstriction and mesangial cell contraction which ↓ RBF > ↓ GFR.
 Renin-Angiotensin = Renin secretion is controlled by (1) intrarenal baroreceptors (2) macula densa (3) renal sympathetic nerves (4) beta-1 activation on granular cells [recall renin degreased angiotensinogen to angiotensin I which is then converted to angiotensin II in the lung].
Renin-Angiotensin = Renin secretion is controlled by (1) intrarenal baroreceptors (2) macula densa (3) renal sympathetic nerves (4) beta-1 activation on granular cells [recall renin degreased angiotensinogen to angiotensin I which is then converted to angiotensin II in the lung].
 Angiotensin II causes (1) vasoconstriction (2) mesangial cell contraction (3) aldosterone secretion (4) increase thirst (5) Na+ reabsorption in the proximal tubule (6) ADH secretion
Angiotensin II causes (1) vasoconstriction (2) mesangial cell contraction (3) aldosterone secretion (4) increase thirst (5) Na+ reabsorption in the proximal tubule (6) ADH secretion
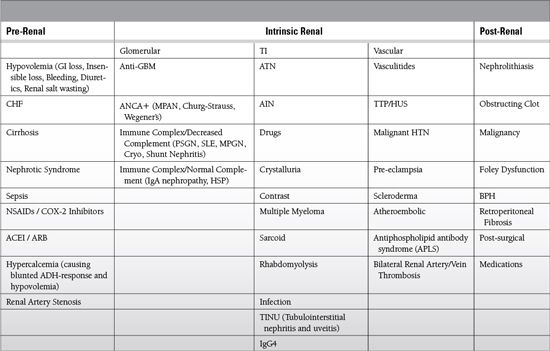
Differential Diagnosis
Diagnosis
History
 Exposures: hypotension, medications, IV contrast, transfusions
Exposures: hypotension, medications, IV contrast, transfusions
 Illness/Injury: surgery, infection, illness, rash
Illness/Injury: surgery, infection, illness, rash
Labs
 BUN/Cr
BUN/Cr
 BUN rising out of proportion to Cr → pre-renal, UGIB, sepsis, corticosteroids, tube feeds
BUN rising out of proportion to Cr → pre-renal, UGIB, sepsis, corticosteroids, tube feeds
 Cr rising out of proportion to BUN → rhabdomyolysis
Cr rising out of proportion to BUN → rhabdomyolysis
 FENa
FENa
 <1% = pre-renal, CIN, pigment nephropathy
<1% = pre-renal, CIN, pigment nephropathy
 >2% = ATN
>2% = ATN
 FEBUN (more useful than FENa if concurrent diuretic use or CKD)
FEBUN (more useful than FENa if concurrent diuretic use or CKD)
 <35% = pre-renal
<35% = pre-renal
 Urine Dipstick
Urine Dipstick
 3+ Protein = consider nephrotic syndrome
3+ Protein = consider nephrotic syndrome
 +Blood, 0 RBCs = consider myoglobinuria
+Blood, 0 RBCs = consider myoglobinuria
 Urine Sediment
Urine Sediment
 Muddy brown casts (epithelial cells) → ATN
Muddy brown casts (epithelial cells) → ATN
 RBC casts / Dysmorphic RBC → GN
RBC casts / Dysmorphic RBC → GN
 WBC casts → AIN (or pyelonephritis)
WBC casts → AIN (or pyelonephritis)
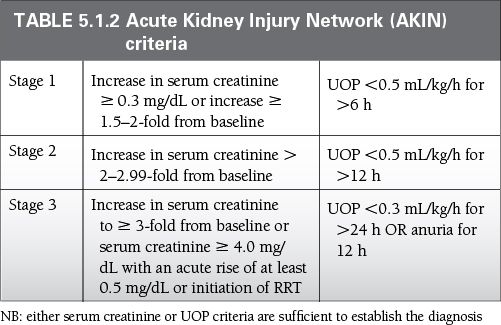
Uniform standards for defining and classifying AKI have been developed by a multidisciplinary collaborative network, and are summarized in Table 5.1.2.
Special Cases
 CK → rhabdomyolysis (polytrauma, crush injury)
CK → rhabdomyolysis (polytrauma, crush injury)
 Uric Acid → tumor lysis (lymphoma, leukemia, metastatic cancer (e.g., melanoma)
Uric Acid → tumor lysis (lymphoma, leukemia, metastatic cancer (e.g., melanoma)
 Urine Eosinophils >1% suggests AIN (sensitivity 40%, specificity 72%, PPV 38%)
Urine Eosinophils >1% suggests AIN (sensitivity 40%, specificity 72%, PPV 38%)
 Peripheral Smear → if schistocytes, consider TTP
Peripheral Smear → if schistocytes, consider TTP
 SPEP + serum FLC → evaluate for multiple myeloma (UPEP only adds minimal value in detection of amyloidosis)
SPEP + serum FLC → evaluate for multiple myeloma (UPEP only adds minimal value in detection of amyloidosis)
 ANA, ANCA, anti-GBM, ASLO, cryocrit, C3/C4 → glomerular disease
ANA, ANCA, anti-GBM, ASLO, cryocrit, C3/C4 → glomerular disease
 Ultrasound → evaluate for hydronephrosis and/or chronicity of renal disease
Ultrasound → evaluate for hydronephrosis and/or chronicity of renal disease
 Electrolytes → monitor for need for RRT
Electrolytes → monitor for need for RRT
Management and Treatment
 Optimize volume status
Optimize volume status
 Support hemodynamics
Support hemodynamics
 Avoid nephrotoxins (contrast, NSAIDs, ACEI/ARB, calcineurin inhibitors, aminoglycosides, fleets enema)
Avoid nephrotoxins (contrast, NSAIDs, ACEI/ARB, calcineurin inhibitors, aminoglycosides, fleets enema)
 Renally dose all medications: antibiotics, opioids, heparin
Renally dose all medications: antibiotics, opioids, heparin
 If serum creatinine rises >1.5 mg/dL in 24 hours assume eGFR<15.
If serum creatinine rises >1.5 mg/dL in 24 hours assume eGFR<15.
Special Cases
 GN: methylprednisolone 0.5 to 1g IV × 3d +/– cyclophosphamide or mycophenolate mofetil +/– plasmapheresis
GN: methylprednisolone 0.5 to 1g IV × 3d +/– cyclophosphamide or mycophenolate mofetil +/– plasmapheresis
 Scleroderma Renal Crisis: titrate captopril to maximum tolerated dose
Scleroderma Renal Crisis: titrate captopril to maximum tolerated dose
 TTP: plasma exchange (consult with blood bank)
TTP: plasma exchange (consult with blood bank)
 Rhabdomyolysis: IVF, IVF, IVF (+/- mannitol, +/- bicarbonate = limited evidence)1
Rhabdomyolysis: IVF, IVF, IVF (+/- mannitol, +/- bicarbonate = limited evidence)1
 AIN: stop offending medications, consider steroids
AIN: stop offending medications, consider steroids
 Drug Crystals: stop drug, alkalinize urine, fomepizole for ethylene glycol
Drug Crystals: stop drug, alkalinize urine, fomepizole for ethylene glycol
 Obstruction: alpha-antagonist, 5alpha-reductase inhibitor, percutaneous nephrostomy
Obstruction: alpha-antagonist, 5alpha-reductase inhibitor, percutaneous nephrostomy
SUGGESTED READINGS
Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: rhabdomyolysis – an overview for clinicians. Crit Care. 2005; 9:158-169.
Mandelbaum T, Scott DJ, Lee J, et al. Outcome of critically ill patients with acute kidney injury using the AKIN criteria. Crit Care Med. 2011;39(12):2659-2664.
Ruffing K, Hoppes P, Blend D, Cugino A, Jarjoura D, Whittier F. Eosinophils in urine revisited. Clin Nephrol. 1994;41(3):163-166.
5.2
Infections of the Urinary Tract
David Stahl
Definitions
 Uncomplicated
Uncomplicated
 Nonpregnant women without structural or neurologic disease (no fever, flank pain, or suspicion of pyelonephritis)
Nonpregnant women without structural or neurologic disease (no fever, flank pain, or suspicion of pyelonephritis)
 Complicated
Complicated
 Upper infection in women, infection in pregnancy, men, patients with neurologic disease, anatomic abnormality, immunosuppression
Upper infection in women, infection in pregnancy, men, patients with neurologic disease, anatomic abnormality, immunosuppression
 Catheter-associated urinary tract infection (CAUTI)
Catheter-associated urinary tract infection (CAUTI)
 3% to 10% risk of infection per day
3% to 10% risk of infection per day
 Prevention: minimize use or intermittent catheterization (by far the most effective), sterile placement, closed collection system
Prevention: minimize use or intermittent catheterization (by far the most effective), sterile placement, closed collection system
 ICU-acquired UTI
ICU-acquired UTI
 Not present on ICU admission or within 2 days of admission
Not present on ICU admission or within 2 days of admission
 Prevalence: 8% to 21%; incidence of 6 to 18.5 per 1,000 catheter days
Prevalence: 8% to 21%; incidence of 6 to 18.5 per 1,000 catheter days
 Risk increased in severe illness, female sex, prolonged duration of catheterization or ICU stay
Risk increased in severe illness, female sex, prolonged duration of catheterization or ICU stay
 Condom or intermittent catheterization has lower rates of UTI in observational studies, but there is no RCT data
Condom or intermittent catheterization has lower rates of UTI in observational studies, but there is no RCT data
Epidemiology
 Overall: Escherichia coli (75% to 95%), Proteus mirabilis, Klebsiella pneumoniae, Staphylococcus saprophyticus
Overall: Escherichia coli (75% to 95%), Proteus mirabilis, Klebsiella pneumoniae, Staphylococcus saprophyticus
 If hematogenous spread suspected Staphylococcus aureus
If hematogenous spread suspected Staphylococcus aureus
 In the ICU
In the ICU
 E. coli(18.5% to 26%), Pseudomonas aeruginosa (10.3% to 16.3%), Enterococcus sp. (14.3% to 17.4%)
E. coli(18.5% to 26%), Pseudomonas aeruginosa (10.3% to 16.3%), Enterococcus sp. (14.3% to 17.4%)
 71% of ICU-acquired UTIs are caused by Gram-negative bacteria.
71% of ICU-acquired UTIs are caused by Gram-negative bacteria.
 Resistance to third-generation cephalosporins being relatively common (20%)
Resistance to third-generation cephalosporins being relatively common (20%)
 Polymicrobial infections are rare: 5% to 12%
Polymicrobial infections are rare: 5% to 12%
 Candida sp. may account for between one-fourth to one-third of ICU-acquired UTI
Candida sp. may account for between one-fourth to one-third of ICU-acquired UTI
Key Pathophysiology
 Usually from migration of bacteria up through urethra
Usually from migration of bacteria up through urethra
 As a result, women have a higher rate of UTIs since they have shorter urethras
As a result, women have a higher rate of UTIs since they have shorter urethras
 Definitions
Definitions
 Lower
Lower
 Urethritis, cystitis
Urethritis, cystitis
 Upper
Upper
 Prostatitis
Prostatitis
 Pyelonephritis: involving renal parenchyma and pelvis (f/c, n/v, diarrhea, flank pain, leukocytosis, pyuria, WBC casts, hematuria)
Pyelonephritis: involving renal parenchyma and pelvis (f/c, n/v, diarrhea, flank pain, leukocytosis, pyuria, WBC casts, hematuria)
 Perinephric abscess: usually 2° ascending infection + preexisting abnormality (stones, anatomy, DM, urologic surgery) → fever, leukocytosis, pain
Perinephric abscess: usually 2° ascending infection + preexisting abnormality (stones, anatomy, DM, urologic surgery) → fever, leukocytosis, pain
 Diagnosis (dx): ultrasound or CT
Diagnosis (dx): ultrasound or CT
Management and Treatment
 Send urine culture and susceptibility prior to initiating treatment.
Send urine culture and susceptibility prior to initiating treatment.
 Consider replacing catheter and sending culture from clean catheter.
Consider replacing catheter and sending culture from clean catheter.
 Uncomplicated cystitis
Uncomplicated cystitis
 Nitrofurantoin, TMP-SMX, fluroquinolones, beta-lactams (with beta-lactamase inhibitor), second-/third-generation cephalosporin
Nitrofurantoin, TMP-SMX, fluroquinolones, beta-lactams (with beta-lactamase inhibitor), second-/third-generation cephalosporin
 AVOID ampicillin or amoxicillin alone (shown to have lower efficacy)
AVOID ampicillin or amoxicillin alone (shown to have lower efficacy)
 Complicated cystitis/pyelonephritis
Complicated cystitis/pyelonephritis
 Treatment to be dictated by degree of illness at presentation, comorbid diseases, resistance patters, and susceptibility data
Treatment to be dictated by degree of illness at presentation, comorbid diseases, resistance patters, and susceptibility data
 Oral
Oral
 Fluoroquinolone (+/– one IV dose, or one dose IV third-generation cephalosporin or one dose IV aminoglycoside—use cephalosporin or aminoglycoside if quinolone resistance known to be >10%)
Fluoroquinolone (+/– one IV dose, or one dose IV third-generation cephalosporin or one dose IV aminoglycoside—use cephalosporin or aminoglycoside if quinolone resistance known to be >10%)
 TMP-SMX (if susceptibility known, or with additional third-generation cephalosporin or aminoglycoside on day 1 if susceptibility unknown)
TMP-SMX (if susceptibility known, or with additional third-generation cephalosporin or aminoglycoside on day 1 if susceptibility unknown)
 Beta-lactam (requires longer course)
Beta-lactam (requires longer course)
 IV
IV
 Fluoroquinolone
Fluoroquinolone
 Aminoglycoside +/− ampicillin
Aminoglycoside +/− ampicillin
 Third-/fourth-generation cephalosporin +/− aminoglycoside
Third-/fourth-generation cephalosporin +/− aminoglycoside
 Extended spectrum penicillin +/− aminoglycoside
Extended spectrum penicillin +/− aminoglycoside
 Carbapenem
Carbapenem
 ICU-acquired UTI
ICU-acquired UTI
 It is difficult to distinguish bacteriuria from UTI in ICU patients.
It is difficult to distinguish bacteriuria from UTI in ICU patients.
 3 to 7 days of appropriate antimicrobial therapy is sufficient for asymptomatic bacteriuria.
3 to 7 days of appropriate antimicrobial therapy is sufficient for asymptomatic bacteriuria.
 Symptomatic CAUTI and/or pyelonephritis should be treated with a change in the catheter and appropriate antimicrobial therapy for 10 to 14 days.
Symptomatic CAUTI and/or pyelonephritis should be treated with a change in the catheter and appropriate antimicrobial therapy for 10 to 14 days.
 ICU-acquired UTI has not been shown to increase mortality and is relatively infrequently associated with bacteremia.
ICU-acquired UTI has not been shown to increase mortality and is relatively infrequently associated with bacteremia.
SUGGESTED READINGS
Al Mohajer M, Darouiche RO. Prevention and treatment of urinary catheter-associated infections. Curr Infec Dis Rep. 2013 Jan. [Epub ahead of print]
Al Raiy B, Jahamy H, Fakih MG, et al. Clinicians’ approach to positive urine culture in the intensive care units. Infect Dis Clin Pract. 2007;15(6):382-384.
Bagshaw SM, Laupland KB. Epidemiology of intensive care unit-acquired urinary tract infections. Curr Op Inf Dis. 2006;19:67-71.
Gaynes R, Edwards JR. Overview of nosocomial infections caused by Gram-negative bacilli. Clin Infect Dis. 2005;41:848-854.
Gupta K, Hooton T, Naber K, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the infectious diseases society of America and the European society for microbiology and infectious diseases. Clin Infect Dis. 2011;52:e103-e120.
Laupland KB, Bagshaw SM, Gregson, DB, et al. Intensive care unit-acquired urinary tract infections in a regional critical care system. Crit Care. 2005;9:R60-R65.
Shuman EK, Chenoweth CE. Recognition and prevention of healthcare-associated urinary tract infections in the intensive care unit. Crti Care Med. 2010; 38(Supp l):S373-S379.
Trautner BW, Darouiche RO. Catheter-associated infections: pathogenesis affects prevention. Arch Intern Med. 2004;164:842-850.
5.3
Acid-Base Physiology and Disorders
David Stahl
Diagnostic Approach
 First: find the primary derangement
First: find the primary derangement
 Second: check for compensatory changes
Second: check for compensatory changes
 pH
pH
 <7.35 = Acidemia
<7.35 = Acidemia
 >7.45 = Alkalemia
>7.45 = Alkalemia
 If acidemia (pH < 7.35)
If acidemia (pH < 7.35)
 PaCO2 > 40 mmHg = Primary respiratory acidosis
PaCO2 > 40 mmHg = Primary respiratory acidosis
 HCO3 < 24 mEq/L = Primary metabolic acidosis
HCO3 < 24 mEq/L = Primary metabolic acidosis
 Anion gap (AG) [Na+ − HCO3− − Cl−] or [Na+ + K+ − HCO3− − Cl−]
Anion gap (AG) [Na+ − HCO3− − Cl−] or [Na+ + K+ − HCO3− − Cl−]
 Increase normal values if included K+
Increase normal values if included K+
 Decrease normal values 2.5 for every 1 mg/dL decrease in albumin
Decrease normal values 2.5 for every 1 mg/dL decrease in albumin
 >12 = Anion gap metabolic acidosis
>12 = Anion gap metabolic acidosis
 Check for osmolar gap
Check for osmolar gap
 Measured Osm − (1.86 * Na+ + glucose/ 18 + BUN/2.8 + ethanol/4.6)
Measured Osm − (1.86 * Na+ + glucose/ 18 + BUN/2.8 + ethanol/4.6)
 >10 mOsm/L look for ingestion
>10 mOsm/L look for ingestion
 Check for excess AG (gap-gap, Δ/Δ) AG − normal AG + measured HCO3
Check for excess AG (gap-gap, Δ/Δ) AG − normal AG + measured HCO3
 >30 = concurrent metabolic alkalosis
>30 = concurrent metabolic alkalosis
 <24 = concurrent non-AG metabolic acidosis
<24 = concurrent non-AG metabolic acidosis
 24–30 = isolated AG metabolic acidosis
24–30 = isolated AG metabolic acidosis
 ≤12 = Non-AGmetabolic acidosis
≤12 = Non-AGmetabolic acidosis
 Check urine AG [UNa + UK − UCl]
Check urine AG [UNa + UK − UCl]
 <0 = extrarenal causes (GI/diarrhea/pancreatic fistulae, NS infusion, RTA Type 2)
<0 = extrarenal causes (GI/diarrhea/pancreatic fistulae, NS infusion, RTA Type 2)
 >0 = renal causes (RTA Type 1 or 4)
>0 = renal causes (RTA Type 1 or 4)
 If alkalemia (pH > 7.45)
If alkalemia (pH > 7.45)
 PaCO2 < 40 mmHg = Primary respiratory alkalosis
PaCO2 < 40 mmHg = Primary respiratory alkalosis
 HCO3− > 24 mEq/L = Primary metabolic alkalosis
HCO3− > 24 mEq/L = Primary metabolic alkalosis
 >20 mEq/L saline unresponsive
>20 mEq/L saline unresponsive
 Excess mineralcorticoid (Conn’s, Cushing’s, steroids, licorice, Liddle’s, Bartter’s, Gitelman’s), milk-alkali, refeeding syndrome
Excess mineralcorticoid (Conn’s, Cushing’s, steroids, licorice, Liddle’s, Bartter’s, Gitelman’s), milk-alkali, refeeding syndrome
 <20 mEq/L saline responsive
<20 mEq/L saline responsive
 Nausea/vomiting (N/V), nasogastric tube (NGT) loss, diuretics, posthypercapnea
Nausea/vomiting (N/V), nasogastric tube (NGT) loss, diuretics, posthypercapnea
Formulae for Compensatory Changes
 Respiratory acidosis
Respiratory acidosis
 Acute
Acute
 ΔpH −0.08 for Δ+10 mmHg pCO2
ΔpH −0.08 for Δ+10 mmHg pCO2
 ΔHCO3− +1 for Δ+10 mmHg pCO2
ΔHCO3− +1 for Δ+10 mmHg pCO2
 Chronic
Chronic
 ΔpH −0.03 for Δ+10 mmHg pCO2
ΔpH −0.03 for Δ+10 mmHg pCO2
 ΔHCO3− +4 for Δ+10 mmHg pCO2
ΔHCO3− +4 for Δ+10 mmHg pCO2
 Respiratory alkalosis
Respiratory alkalosis
 Acute
Acute
 ΔpH +0.08 for Δ−10 mmHg pCO2
ΔpH +0.08 for Δ−10 mmHg pCO2
 ΔHCO3− −2 for Δ−10 mmHg pCO2
ΔHCO3− −2 for Δ−10 mmHg pCO2
 Chronic
Chronic
 ΔpH +0.03 for Δ−10 mmHg pCO2
ΔpH +0.03 for Δ−10 mmHg pCO2
 ΔHCO3− −5 for Δ−10 mmHg pCO2
ΔHCO3− −5 for Δ−10 mmHg pCO2
 Metabolic acidosis (if spontaneous respiration)
Metabolic acidosis (if spontaneous respiration)
 Δ−1 mmHg pCO2 Δ−1 HCO3−
Δ−1 mmHg pCO2 Δ−1 HCO3−
 Metabolic alkalosis (if spontaneous respiration)
Metabolic alkalosis (if spontaneous respiration)
 Δ−7 mmHg pCO2 Δ−10 HCO3−
Δ−7 mmHg pCO2 Δ−10 HCO3−
Strong Ion Difference/Stewart Model
 Stewart derived three independent variables to explain acid-base physiology based on blood plasma:
Stewart derived three independent variables to explain acid-base physiology based on blood plasma:
 Strong ion difference (SID)
Strong ion difference (SID)
 Strong ions are derived from compounds that fully dissociate at physiologic pH.
Strong ions are derived from compounds that fully dissociate at physiologic pH.
 The SID is the difference between the sum of the concentrations of all dissociated cations and all dissociated anions, and is roughly equal to 40 mEq/L.
The SID is the difference between the sum of the concentrations of all dissociated cations and all dissociated anions, and is roughly equal to 40 mEq/L.
 SID = [Na+] + [K+] + [Ca2+] + [Mg2+] − [Cl−] − [Xa−]
SID = [Na+] + [K+] + [Ca2+] + [Mg2+] − [Cl−] − [Xa−]
 SID can be approximated as [Na+] + [K+] − [Cl−], where [Xa−] represents other unmeasured strong anions.
SID can be approximated as [Na+] + [K+] − [Cl−], where [Xa−] represents other unmeasured strong anions.
 Weak acids [Atot−]
Weak acids [Atot−]
 Strong acids [HB] completely dissociate at physiologic pH into [H+] and [B−].
Strong acids [HB] completely dissociate at physiologic pH into [H+] and [B−].
 Weak acids [HA] only partially dissociate into [H+] and [A−].
Weak acids [HA] only partially dissociate into [H+] and [A−].
 The sum of the concentrations of these weak acids is represented as [Atot−].
The sum of the concentrations of these weak acids is represented as [Atot−].
 These compounds represent the buffer activity of the system including proteins (primarily albumin), sulfates, and phosphates.
These compounds represent the buffer activity of the system including proteins (primarily albumin), sulfates, and phosphates.
 PaCO2
PaCO2
 Links the metabolic and respiratory processes where dissolved plasma CO2 is regulated by ventilation.
Links the metabolic and respiratory processes where dissolved plasma CO2 is regulated by ventilation.
 In this model [H+] and pH are dependent variables derived from the above independent variables (primarily SID).
In this model [H+] and pH are dependent variables derived from the above independent variables (primarily SID).
 Deficit or excess of water in plasma will concentrate or dilute the strong cations and anions equally and therefore increase or reduce the SID.
Deficit or excess of water in plasma will concentrate or dilute the strong cations and anions equally and therefore increase or reduce the SID.
 This model may more effectively account for acidosis (excess of unmeasured anions such as lactate or ketones) that would otherwise be obscured by the alkalinizing effect of hypoalbuminemia (deficit of weak acid) commonly seen in ICU patients, but is not commonly used, nor has it been shown to affect clinical outcomes.
This model may more effectively account for acidosis (excess of unmeasured anions such as lactate or ketones) that would otherwise be obscured by the alkalinizing effect of hypoalbuminemia (deficit of weak acid) commonly seen in ICU patients, but is not commonly used, nor has it been shown to affect clinical outcomes.
 Check urine Cl
Check urine Cl


