Edited by Anne-Maree Kelly Steve Goodacre Chest pain is one of the most common presenting complaints in emergency medicine. It is also associated with life-threatening pathology, so it is arguably the most important complaint faced by emergency clinicians. It is certainly one of the most challenging. Failure to diagnose and manage appropriately patients with acute chest pain is a frequent cause of avoidable mortality and morbidity and is a leading cause of malpractice litigation. It is therefore not surprising that physicians often err on the side of caution, yet this can also have adverse consequences for the patient and society. Patient anxieties following unnecessary investigation are often unrecognized but may severely affect quality of life, while over-investigation and unnecessary hospital admission for chest pain waste millions of healthcare dollars each year. The incidence of acute chest pain presenting to the emergency department appears to be increasing. Awareness of the importance of early treatment for myocardial infarction has led to public information campaigns that increase emergency department attendances with chest pain. Meanwhile, general practitioners are increasingly being bypassed in favour of an emergency ambulance response. These changes in health service use have coincided in many developed countries with a decrease in the incidence of coronary heart disease. It therefore seems likely that patients presenting to the emergency department with acute chest pain have decreasing prevalence of acute coronary syndrome (ACS) and increasing prevalence of more benign conditions. The main differential diagnoses are outlined in Table 5.1.1. The most common causes of acute chest pain are ACS (unstable angina or myocardial infarction), musculoskeletal pain, anxiety, gastro-oesophageal pain and non-specific chest pain. The most serious causes (in terms of threat to life) are ACS, pulmonary embolism and aortic dissection. Since ACS is both common and life threatening, it is inevitably the primary focus of assessment. Table 5.1.1 ACS is discussed in detail in Chapter 5.2, pulmonary embolus in Chapter 5.5 and aortic dissection in Chapter 5.10. Musculoskeletal chest pain may be related to a precipitating episode, such as chest wall injury or physical over-exertion. Alternatively, it may be caused by inflammation in chest wall structures. Tietze’s syndrome (costochondritis) is most commonly seen in women and is characterized by tenderness of the costochondral cartilages. Epidemic myalgia (Bornholm disease) is due to inflammation of chest wall muscles and pleura occurring after viral infection, typically Coxsackie B. Herpes zoster produces severe pain along the distribution of a thoracic nerve and may be misdiagnosed as musculoskeletal pain if the patient presents before any rash or vesicles have developed. Gastro-oesophageal pain occurs when gastric contents reflux into the oesophagus or when the oesophageal muscles spasm. Pneumomediastinum can occur spontaneously after vigorous exercise, vomiting or an asthma attack or may be associated with barotrauma from diving or inhalation during drug abuse. Pericarditis is most commonly caused by viral infection, but may be associated with systemic illness, such as uraemia or autoimmune disease, or follow myocardial infarction or cardiac surgery (Dressler syndrome). Anxiety-related chest pain is a common and frequently unrecognized cause of acute chest pain. It may also coexist and be an important factor alongside other causes of chest pain. The patient with coronary heart disease and anxiety-related chest pain presents a particularly difficult diagnostic and management challenge. Anxiety may be related to a specific serious cause of chest pain and can be exacerbated by misguided efforts to provide reassurance through diagnostic testing. In extreme cases, this can lead to ‘cardiac neurosis’ in which the patient’s anxieties about cardiac disease cause more severe disruption to their daily activities and quality of life than would be expected from the pathology that worries them. Pleurisy is typically caused by a viral infection and produces pain that is worse on inspiration. It may be differentiated from pulmonary embolus by the presence of systemic features and the absence of breathlessness or risk factors for thromboembolism, although investigation for pulmonary embolism is often required. Pneumonia and pneumothorax can also cause pleuritic pain but should be evident on chest radiography. There are a number of serious abdominal complaints that may present as chest pain. These include biliary colic (acute biliary pain), cholecystitis, peptic ulcer disease and pancreatitis. Failure to take a careful history and examine the abdomen may lead to delayed diagnosis. Finally, a substantial proportion of patients will be labelled entirely appropriately as ‘non-specific chest pain’ after emergency department evaluation. These patients have pain that simply cannot be categorized into a clear diagnostic group. It is more honest to accept this than apply an inaccurate diagnostic label. Clinical assessment is primarily aimed at identifying patients with a significant risk of serious pathology who require further investigation and possibly inpatient care. The most common serious pathology is ACS, so clinical assessment is often focused upon associated features. Other serious conditions, such as pulmonary embolism and aortic dissection, should not be neglected. ACS is classically associated with chest pain that is crushing, gripping or squeezing in nature and radiates to the left arm, but presenting features in the emergency department may be much more variable, particularly in patients with no past history of coronary heart disease and a non-diagnostic ECG. Table 5.1.2 shows the likelihood ratios of clinical features that may help to diagnose ACS. It is notable that pain radiating to the right arm or to both arms is a powerful predictor of ACS. Pain described as ‘burning’ or ‘like indigestion’ can be associated with ACS, as can pain occurring on exertion. So the diagnoses of gastro-oesophageal reflux or stable angina should be made with great caution. Pain that is sharp or associated with inspiration or movement is less likely to be cardiac, but these findings alone do not exclude ACS. Risk factors for coronary heart disease should be routinely recorded, although they may have surprisingly little diagnostic value. This is perhaps because patients are aware of these risk factors and take them into account when deciding whether or not to seek help for episodes of chest pain. In this respect, social and cultural factors may have an important influence upon patient’s interpretation of their symptoms and health-seeking behaviour. Table 5.1.2 Likelihood ratios of clinical features useful for diagnosing acute myocardial infarction Clinical examination is of limited diagnostic value and is mainly aimed at identifying non-cardiac causes of chest pain or complications of ACS, such as arrhythmia, heart failure or cardiogenic shock. Pain that can be reproduced by chest wall palpation is less likely to be cardiac, but this finding does not exclude the possibility of ACS. It is also important to determine specifically that chest wall palpation is reproducing the pain that led to presentation. Simply identifying chest wall tenderness has little value – everyone has a tender chest wall if you press hard enough! Clinical assessment should not just focus upon ACS, but should aim positively to identify other causes. Pulmonary embolism is diagnostically challenging. Suspicion should be raised by chest pain that is clearly pleuritic in nature, haemoptysis, associated breathlessness, features of deep vein thrombosis or risk factors for venous thromboembolism (immobilization, malignancy, recent trauma or surgery, pregnancy, intravenous drug abuse or previous thromboembolism). Clinical examination may reveal tachycardia, tachypnoea or features of deep vein thrombosis (see Chapter 5.5). Aortic dissection is characterized by severe pain radiating to the back with associated diaphoresis. Neurological symptoms or signs, sometimes transient, are common. Clinical examination may reveal discrepancy between blood pressure in right and left arms (see Chapter 5.10). Clinical assessment of chest pain should always include examination of the abdomen to identify tenderness, guarding, rebound tenderness or a positive Murphy’s sign. Unnecessary investigation can be avoided if non-life-threatening pathology can be confidently diagnosed by clinical assessment. Pain that is reproduced by chest wall palpation in a patient at low risk of coronary heart disease and with no significant risk factors for pulmonary embolus can be confidently diagnosed as musculoskeletal. A positive diagnosis is particularly valuable for the patient who is primarily suffering from anxiety-related symptoms. In this case, pain is typically described as tightness around the chest and associated with a feeling of restricted breathing. Other features include palpitations (particularly awareness of the heartbeat), sweating, breathlessness, light-headedness, feelings of panic, or paraesthesia of the lips or fingertips. The ECG is the most useful clinical investigation and should be performed on all patients presenting with acute non-traumatic chest pain. Table 5.1.3 shows the value of ECG features for diagnosing myocardial infarction. It is important to recognize that a normal ECG does not rule out myocardial infarction. ST-segment elevation or depression, new Q waves and new conduction defects are specific for acute myocardial infarction and predict adverse outcome. Patients with these features should be managed on a coronary care unit. Other changes associated with myocardial infarction are less helpful. T-wave changes are often non-specific and may be positional or due to numerous other causes. ECG changes in pulmonary embolism are also non-specific. Table 5.1.3 Likelihood ratios of ECG features useful for diagnosing acute myocardial infarction A standard 12-lead ECG may be augmented by serial ECG recording or continuous ST-segment monitoring. These may detect evolving ECG changes or dynamic ST-segment changes. However, these techniques may also identify non-specific false-positive changes, such as minor T-wave inversions, especially if they are used inappropriately in patients with a low risk of coronary heart disease. ST-segment monitoring was developed for the high-risk coronary care population; in low risk emergency department patients with chest pain, it has a very low yield of significant findings. Like clinical examination, the chest radiograph is mainly intended to identify non-cardiac causes for chest pain, such as a pneumothorax or fractured rib and complications of myocardial infarction, such as left ventricular failure. Although it is often routinely ordered, it is not usually helpful. Cardiac biomarkers are key investigations in acute chest pain and are a source of much heated debate. They are also a progressively developing technology, so this chapter will focus upon the principles that should guide their use. Three key features determine the clinical value of a cardiac biomarker. Sensitivity tells us how good the marker is at identifying patients with disease, and thus how useful it is for ruling out myocardial ischaemia. Specificity tells us how good the marker is at identifying patients without disease, and thus how useful it is for ruling in myocardial ischaemia (i.e. a specific test that is positive suggests that the patient is very likely to have ischaemia). The prognostic value (often expressed as a relative risk) tells us how good the marker is at predicting future adverse events, such as death, myocardial infarction or life-threatening arrhythmia. Intuitively, clinicians tend to be most concerned about sensitivity. If a marker lacks sensitivity then it may miss cases of myocardial infarction leading to potentially catastrophic discharge home without appropriate treatment. However, sensitivity and specificity are often related and may be influenced by the threshold of the marker used to determine a positive test. The lower the threshold used for a positive test, the higher the sensitivity and the lower the specificity. Many evaluations of new markers deliberately optimize sensitivity by selecting a low threshold and sacrificing specificity. This may be an acceptable trade-off in a high-risk population, but emergency department patients with no past history of coronary heart disease and a non-diagnostic ECG typically have a low prevalence of myocardial infarction (<10%). In these circumstances, a test with low specificity will generate many false-positive results requiring hospital admission and investigation, as well as unnecessary anxiety for the patient. Furthermore, it should be remembered that even a random process can be made to appear sensitive by setting a low diagnostic threshold so that most of the results generated are deemed positive. For example, rolling a pair of dice to diagnose myocardial infarction will have 97% sensitivity if all results, except double six, are deemed to be positive. This is, of course, an extreme example, but biomarkers with diagnostic value that is little better than rolling dice have been promoted by studies that report high sensitivity while hiding away poor specificity. Sensitivity should always be reported with a corresponding specificity. The prognostic value of a marker is arguably even more useful than its diagnostic parameters, particularly if the marker can predict high-risk patients who will benefit from treatment. If a prognostically useful marker is positive, then we know the patient has the potential to benefit from intervention; if it is negative then we know that, even if further investigation is required to identify the exact cause of their chest pain, they are unlikely to benefit from hospital admission and treatment. Prognostic considerations explain changes in the definition of myocardial infarction. The original World Health Organisation (WHO) definition of myocardial infarction was based upon creatine kinase, a cardiac marker with limited sensitivity and specificity and only weak evidence of an association with adverse prognosis. Subsequent definitions of myocardial infarction, from the American Heart Association and European Society of Cardiology, used troponin as the biomarker based on research showing elevated troponin levels were associated with increased risk of adverse outcome. Furthermore, research has shown that informing clinicians that their patient has an elevated troponin level can alter patient management and reduce the risk of major adverse cardiac events over the following year. This is important evidence that measuring troponin provides patient benefit by reducing adverse outcomes. Creatine kinase is released by damaged myocardium, but is also released by muscles and the liver and is measurable in the blood in the absence of pathology. Its MB isoenzyme (CK-MB) is more cardiac-specific but shares the same problems. Substantial myocardial damage is required to produce an elevated CK-MB, but CK-MB may also be elevated in the absence of myocardial injury. Its role in diagnosis is generally limited to situations where troponin testing is unavailable or likely to be unreliable. There are two troponin assays, troponin I and troponin T, with little to choose between them in terms of diagnostic or prognostic performance. The diagnosis of myocardial infarction is based upon elevation of the troponin level above the 99th percentile upper reference limit of a normal reference population. This value will vary between assays. The precision of the assay is described by the coefficient of variation (CV) at the 99th percentile. There have been several generations of troponin assays with newer assays having higher sensitivity and better precision. High-sensitivity troponin assays have optimal precision (≤10% CV) at the 99th percentile and can detect myocardial infarction earlier after symptom onset than other assays. However, this appears to be at the cost of an increased number of low troponin rises that are of unclear diagnostic and prognostic significance. Troponin elevation is not specific for myocardial infarction and low levels measured using a high sensitivity assay may not indicate significant pathology. Even substantial troponin elevations may not be due to ACS. Troponin can be elevated in pulmonary embolus, sepsis, renal failure, congestive cardiac failure and a number of other illnesses. The use of troponin has tended to be limited by its lack of early sensitivity. It has been estimated that standard troponin assays take up to 12 hours after symptom onset to achieve optimal sensitivity, so if it is used too early after symptom onset it may produce a false-negative result. This has led to the widespread practice of delaying troponin measurement to at least 12 hours after symptom onset to achieve optimal sensitivity. This practice is problematic because most patients present a few hours after symptom onset, so enforcing a 12-hour delay will typically require hospital admission or use of observation facilities. If there is limited availability of such facilities, clinicians may feel under pressure to discharge the patient without any testing. Thus, a strategy intended to increase patient safety may paradoxically put patients at risk when applied to the real world. Newer assays allow a 6-hour testing strategy and recently developed high-sensitivity assays appear to have good early sensitivity and may be able to rule out myocardial infarction within a few hours of presentation to hospital. However, as mentioned above, this may be at the cost of limited specificity resulting in the generation of troponin elevations that have little diagnostic or prognostic significance. Thus, the choice of testing strategy involves a trade-off between sensitivity and specificity. It is important to know the test used in your institution and its performance characteristics in order to decide on an appropriate serial testing strategy. Awareness of the limited early sensitivity of standard troponin assays has led to research into alternative biomarkers that can detect myocardial infarction in the initial hours after symptom onset. These biomarkers often have poor specificity so any improvement in sensitivity achieved by combining troponin with another biomarker involves a trade-off in terms of reduced specificity. The development of high-sensitivity troponin assays is likely to undermine the need to develop and use alternative early biomarkers. Future evaluations of alternative early biomarkers will need to include comparison with high-sensitivity troponin. The most extensively evaluated early biomarkers to date are myoglobin, heart-type fatty acid binding protein (HFABP) and ischaemia modified albumen (IMA). Table 5.1.4 shows the sensitivity and specificity of these biomarkers at presentation to hospital, compared to troponin. None of the alternative biomarkers have sufficient sensitivity to rule out myocardial infarction at presentation. Adding HFABP or myoglobin to a contemporary troponin assay at presentation increases sensitivity at the expense of specificity, but it is not clear whether either biomarker can improve the early sensitivity of a high-sensitivity troponin assay. IMA appears to have insufficient specificity to play any useful role in diagnosis. Table 5.1.4 Sensitivity and specificity of biomarkers for myocardial infarction at presentation to hospital The estimates are based on meta-analysis of data from heterogeneous studies and are therefore subject to substantial uncertainty. Goodacre S, Thokala P, Carroll C, Stevens J, Leaviss J, et al. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess 2013;17(1) http://www.journalslibrary.nihr.ac.uk/hta/volume-17/issue-1 with permission. *All assays combined, using 99th percentile as a diagnostic threshold. Biomarkers are continually being developed and emergency physicians can expect to see headline-grabbing publications extolling their virtues. However, they should be wary before indiscriminately using new markers in their patients with chest pain. As described earlier, the emergency department population with chest pain are a heterogeneous population with a relatively low prevalence of ACS compared to the high-risk patients that usually comprise research study populations. Indiscriminate use of markers with limited specificity will lead to many false-positive results and consequent patient anxiety, unnecessary investigation and waste of resources. Provocative cardiac testing, usually using an exercise treadmill, has been used in a number of emergency departments. Patients typically undergo a short period of observation and cardiac marker testing to rule out myocardial infarction before receiving an exercise treadmill test. Concerns about the safety of this procedure have been addressed by data from a number of centres, but it should be recognized that selection of low-risk patients plays a key role in ensuring safety. Exercise treadmill testing has relatively poor sensitivity and specificity and cannot reliably rule in or rule out coronary artery disease. However, it is prognostically useful and predicts risk of adverse events over the months following attendance. It is therefore used to risk-stratify more than to diagnose. A patient with a negative treadmill test may have coronary artery disease but can be reassured that they are at low risk of adverse outcome. It is not clear whether the additional prognostic information provided by exercise treadmill testing justifies widespread use. CT coronary angiography is developing an increasing role in the diagnostic evaluation of acute chest pain. Unlike exercise treadmill testing, CT coronary angiography detects coronary obstruction with a reasonable degree of accuracy. Sensitivity and specificity are both around 90% against a reference standard of invasive coronary angiography. However, coronary atheroma is a common finding and detecting atheroma, or even coronary obstruction, does not necessarily mean that this was the cause of the patient’s chest pain. Studies of the prognostic value of CT coronary angiography in patients with suspected ACS have been limited by selection of patients with low rates of adverse outcome and have, as yet, failed to show worthwhile prognostic value. This means that, although CT coronary angiography is a promising diagnostic test for acute chest pain, evidence is lacking that its use improves patient outcomes and its role in routine assessment remains unclear. The combination of observation and cardiac marker testing to rule out myocardial infarction, followed by provocative cardiac testing to risk-stratify or CT coronary angiography to diagnose coronary artery obstruction, has been adopted in many hospitals in the form of a chest pain unit or pathway. These have a number of potential benefits for patients and health services and some evidence to suggest that they reduce the probability of admission, reduce the risk of discharge with ACS, improve patient satisfaction and quality of life and reduce health service costs. However, as an organizational intervention, the effect of the chest pain unit will depend upon local circumstances and may be influenced by staff attitudes, professional roles and local leadership. Furthermore, the presence of a chest pain unit may attract additional attendances with chest pain. Whether this represents identification of unmet demand or unnecessary work is a matter of opinion. A number of clinical risk scores have been developed to risk-stratify patients with suspected ACS. The Goldman algorithm and acute cardiac ischaemia time insensitive predictive instrument (ACI-TIPI) were developed and validated on large cohorts of patients with chest pain in the 1980s and 1990s. The Goldman algorithm uses a series of questions about the patient’s age, clinical history and ECG findings to categorize patients into a low (<7%) or high (>7%) risk of myocardial infarction, based on the WHO definition used at the time. ACI-TIPI can be incorporated into a computerized ECG. The user enters the patient’s age, sex and whether chest or left arm pain is the primary symptom. The computer then uses these data and analysis of the ECG to generate a probability of acute cardiac ischaemia. The thrombolysis in myocardial infarction (TIMI) score has been developed and validated as a predictor of adverse outcome in patients with diagnosed ACS (see Chapter 5.2). Studies have evaluated the TIMI score in emergency department patients with suspected ACS and shown that higher scores are associated with a higher risk of adverse outcome. This has led to the TIMI score being used to risk stratify patients with chest pain before a diagnosis of ACS has been confirmed. The ECG and cardiac biomarker components are the most powerful predictors among the elements of the TIMI score and a modified version of the TIMI score, with extra weighting for these elements, has been proposed for patients with acute chest pain. It is not clear whether either the original or modified TIMI score provides better prognostic information than that provided by ECG and biomarkers alone. The Global Registry of Acute Coronary Events (GRACE) freedom-from-event score has recently been developed to predict adverse events in people with non-ST-elevation acute coronary syndrome and can identify up to 30% of the admitted population who are at low risk of death or any adverse in-hospital event. It is more complex to calculate than the TIMI score but bedside calculation can be easily facilitated by mobile technologies. The GRACE freedom-from-event score offers an alternative to the TIMI score for predicting adverse outcome but further validation in the wider chest pain population is required. Treatment of acute chest pain is directed at the specific cause. The treatment of ACS is outlined in Chapter 5.2, pulmonary embolus in Chapter 5.5 and aortic dissection in Chapter 5.10. Musculoskeletal chest pain, whether due to muscular strain, chest wall injury, Tietze’s syndrome or epidemic myalgia, should be treated with simple analgesia and the patient advised to see their general practitioner if the pain persists beyond a few weeks. It is also worth considering whether anxiety may be exacerbating the patient’s symptoms. Gastro-oesophageal pain can be treated acutely by antacids, although the diagnostic value of observing relief of pain with antacids (the so-called ‘GI cocktail’) is debatable. ACS can present as burning or indigestion-type pain and, pain being typically fluctuant, may ease coincidentally with administration of an antacid. Gastro-oesophageal pain should be diagnosed with caution and ideally only after ACS has been investigated and ruled out. In these circumstances, a course of treatment with a proton pump inhibitor is appropriate. Follow up will depend upon local practice along with the duration and severity of symptoms. Anxiety-related symptoms range from simple chest wall muscular tension to panic attacks, hyperventilation syndrome and cardiac neurosis. Treatment should therefore be tailored to the patient’s individual needs. In many cases, anxiety will be an understandable reaction to concerns about heart disease or other serious pathology. The first step is therefore to provide clear and unequivocal reassurance. If diagnostic uncertainty makes this impossible then it may still be possible to provide reassurance by highlighting the excellent prognosis of patients with chest pain whose tests are negative. Information leaflets have been shown to reduce anxiety after emergency department attendance with chest pain. Patients with more severe symptoms may benefit from relaxation techniques, cognitive behavioural therapy or treatment with an antidepressant. These are best arranged through the patient’s general practitioner. Managing anxiety in the emergency department patient is often complicated by difficulties in satisfactorily ruling out serious physical illness. A diagnosis of anxiety may be considered likely, but until cardiac testing is complete (perhaps even involving coronary angiography), the treating physician may be reluctant to discuss treatment of anxiety with the patient. This is inappropriate. If the patient has significant anxiety-related symptoms then this will adversely affect their quality of life and should be addressed regardless of whether they ultimately also need treatment for cardiac disease. Non-specific chest pain obviously presents a challenge. With no clear diagnosis it is difficult to advise an appropriate treatment. However, patients can be advised that, although no clear diagnosis can be made, about half such patients presenting to the emergency department have no further episodes of pain over the following month. Those who do suffer further episodes of pain are unlikely to be troubled. Treatment is therefore unlikely to be required. Finally, an acute episode of chest pain provides an opportunity to identify and manage cardiac risk factors at a time when the patient is likely to be most receptive to lifestyle advice. Smokers should be advised to use the episode as a stimulus to stop smoking and referral to a smoking cessation service arranged. General dietary and exercise advice may also be helpful. Blood pressure, blood glucose and lipid profile may be requested as part of clinical assessment, although any abnormalities identified should preferably be referred to the patient’s general practitioner, who will be best placed to provide overall cardiovascular risk assessment, intervention and long-term follow up. Prognosis will also depend upon the underlying pathology and the prognoses of various causes of chest pain are discussed in the relevant chapters of this book. Patients with no obvious diagnosis after clinical assessment, ECG and troponin testing have an excellent prognosis. There is some evidence that patients who attend the emergency department with chest pain have a higher risk of adverse cardiac events than the general population, even if cardiac disease is ‘ruled out’ at initial presentation, but this risk is not high enough to warrant active intervention beyond ensuring that any cardiac risk factors identified have been addressed. Chest pain is responsible for a substantial and growing number of emergency medical admissions in many countries. This is placing a major burden upon healthcare systems. The value of hospital admission for low risk ACS and pulmonary embolus is being questioned and it is likely that there will be increasing efforts to manage acute chest pain without admission to hospital. These efforts may be successful for younger patients with no co-morbidities and a single potentially serious cause for their chest pain, but may be difficult to implement among the growing population of older patients with co-morbidities or multiple potentially serious causes for their pain. High-sensitivity troponin assays are increasingly replacing other contemporary assays. This will lead to more positive results and concerns that some of these may represent false-positive results with little prognostic value. Emergency physicians may therefore need to be more circumspect in their use of troponin and avoid measuring troponin in patients with a very low risk of ACS in whom an elevated high-sensitivity troponin measurement just above the diagnostic threshold is likely to cause confusion rather than provide a clear diagnosis. Point-of-care troponin testing has been available for some time but widespread adoption has been limited by a number of factors, including lack of usability and difficulties converting reduced turnaround times into reduced length of hospital stay. The development of hand-held point-of-care machines using finger-stick capillary blood samples could improve the usability of point-of-care testing and extend use to the pre-hospital setting. CT coronary angiography is likely to be more widely used in the assessment of acute chest pain. In the absence of randomized trial evidence of effectiveness, it will not be clear whether patients are benefitting from increased use. Both high sensitivity troponin assays and CT coronary angiography have the potential to increase the diagnostic yield of positive tests in the investigation of acute chest pain leading to over-burdening of cardiology services. Chest pain management is likely to be influenced by changes in health service policy, which are, in turn, likely to depend upon local social, political and economic factors. These changes will be variable and may be unpredictable. On one hand, public awareness of the medical significance of chest pain and policies aimed at increasing rapid access to care may lead to increased numbers of patients presenting with chest pain. On the other hand, reorganization of services and attempts to control costs may result in an opposite effect. Specifically, development of primary angioplasty services may lead to centralization of chest pain services and patients bypassing facilities that do not provide primary angioplasty. Steve Goodacre and Anne-Maree Kelly Acute coronary syndrome (ACS) is the most common life-threatening condition in emergency medicine. Failure to identify and treat it promptly risks avoidable morbidity and mortality. ACS nearly always occurs as a consequence of atheroma in the coronary arteries, commonly known as coronary heart disease (CHD). Many people have coronary atheroma but are asymptomatic because it is not extensive enough to occlude coronary blood flow. Others have a degree of coronary occlusion that does not cause symptoms unless they exert themselves or if myocardial oxygen demand is increased by some other mechanism, such as anaemia. Cardiac chest pain that only occurs on exertion and is rapidly relieved by rest is known as stable angina and is not classified as an ACS. ACS usually occurs when an atheromatous plaque ruptures or fissures. Haemorrhage may occur into the plaque or thrombus may accumulate over the fissure. The type of ACS that results depends on the extent of the rupture and degree of haemorrhage or thrombus formation. A gradually progressive occlusion will usually produce symptoms of unstable angina: progressive symptoms of myocardial ischaemia occurring on less exertion or at rest. A rapidly progressive occlusion may lead to myocardial infarction (MI), with severe pain at rest and the potential for serious complications, such as arrhythmia, heart failure, cardiogenic shock or sudden cardiac death. If coronary occlusion is minor or transient, the consequent myocardial ischaemia will not lead to myocardial damage. If coronary occlusion is severe or prolonged then myocardial necrosis will occur. Not all coronary artery occlusion is due to coronary atheroma. Prinzmetal angina describes a syndrome in which myocardial ischaemia is associated with coronary artery spasm and is characterized by transient ST-segment elevation on the electrocardiograph (ECG). Coronary angiography may show minor atheroma or normal coronary arteries. Uncommonly, coronary artery spasm may be severe enough to cause myocardial necrosis and an associated biomarker elevation. Other rare causes of coronary artery occlusion include Kawasaki’s disease, in which occlusion is due to inflammation in the coronary artery and aortic dissection that involves the coronary arteries. ACS may involve occlusion of one or more of the coronary arteries and the location of occlusion may determine the clinical presentation, ECG findings and likelihood of complications. The most common site for MI is the anterior or anteroseptal region. It usually results from occlusion of the left anterior descending artery. It has a worse prognosis than other types of MI and complications are more common. Sudden cardiac death may result from total occlusion of the left anterior descending artery, giving a lesion in this location the grim sobriquet of ‘widow-maker’. Lateral infarction is usually caused by occlusion of the circumflex artery or the diagonal branch of the left anterior descending artery. Inferior MI is usually caused by occlusion of the right coronary artery or the circumflex artery. It has a better prognosis than anterior infarction and ventricular dysfunction is less likely, although heart block due to involvement of the atrioventricular node is more common. Posterior infarction is usually due to occlusion of the right coronary artery or, less commonly, the circumflex artery in patients with dominance of the left coronary circulation. Posterior or inferior MI may result in right ventricular infarction leading to right ventricular failure. ACS may be associated with a range of life-threatening complications, including arrhythmia, such as atrial fibrillation, ventricular tachycardia and ventricular fibrillation. Supraventricular tachycardias are not usually associated with ACS. Heart block may occur with infarcts affecting the nodal branch of the right coronary artery or septal infarcts. Infarction may lead to myocardial dysfunction, resulting in heart failure or cardiogenic shock. Uncommon complications include papillary muscle dysfunction and mitral regurgitation, ventricular septal defect or cardiac rupture. The probability of any of these complications increases with the severity of myocardial damage incurred. Coronary heart disease is the leading cause of death in the world. It is responsible for 6.8% of disability-adjusted life years (DALYs) lost through disease by men and 5.3% of DALYs lost by women. The global burden of CHD is expected to rise from 47 million DALYs in 1990 to 82 million in 2020. Most of this increased burden will be in developing countries. However, CHD mortality rates have dramatically decreased in many developed countries since the 1980s. Studies suggest that 50–75% of the fall in cardiac deaths can be attributed to population interventions, particularly those relating to smoking, hypertension and high cholesterol. The remaining 25–50% is due to treatments for patients with CHD, such as acute reperfusion therapies (including thrombolysis and percutaneous coronary intervention (PCI)), aspirin, angiotensin-converting enzyme inhibitors, statins and coronary artery bypass surgery. The main risk factors for CHD are well established and include smoking, diabetes, hypertension, hyperlipidaemia and a family history of CHD at a young age, while obesity and lack of exercise may play a contributory role. Age and sex are also important. CHD prevalence increases with age and increases at an earlier age (40–50 years) in men than in women (over 60 years). Everyone over the age of 60 is effectively at risk of CHD. Conversely, a history of CHD presenting in a relative when they were aged over 60 should not be considered a significant risk factor. Patients presenting to the emergency department (ED) with chest pain in general, and ACS specifically, show a diurnal variation with a peak of attendances during the morning, although many of these attendances relate to symptoms occurring overnight. Presentation is more common on a Monday, when cardiovascular mortality appears to be higher. Cardiovascular mortality also increases during the winter months, particularly in colder climates. Prevention of ACS is achieved principally by preventing underlying CHD, although secondary prevention of ACS in patients with established CHD can be attempted by ensuring appropriate treatment with daily low-dose aspirin, angiotensin-converting enzyme (ACE) inhibitor, β-blockers and lipid-lowering therapy. Primary CHD prevention can take place by addressing the important coronary artery disease risk factors that are amenable to intervention. The most important modifiable risk factors at a population level are smoking, obesity and lack of exercise. These may be tackled by legislation and education and by economic and social policy. Diabetes, hypertension and hyperlipidaemia can be addressed at an individual level. It is increasingly recognized that the importance of any one risk factor depends on the presence of other risk factors, so cardiovascular risk is most appropriately assessed by a comprehensive assessment involving all risk factors, along with age and sex. Screening programmes should be based on overall cardiovascular risk assessment, rather than individual risk factors. Similarly, the decision to prescribe treatments for risk factors, particularly lipid-lowering therapy, should be based on overall cardiovascular risk. This has implications for emergency medicine. It may be tempting to use the patient’s attendance at the ED to undertake opportunistic screening by, for example, measuring blood pressure, blood sugar or lipids, even though they will not influence management of the presenting complaint. This approach is inappropriate because it does not involve overall cardiovascular risk assessment. Furthermore, it may be considered unethical because the patient is effectively being screened (with potential implications for health insurance) without the opportunity to make an informed choice about whether they wish to receive screening. For these reasons, coronary risk assessment for primary prevention is best left to primary care physicians. Although opportunistic screening in the ED is best avoided, opportunistic patient education about risk factors may be very salient, particularly if the patient has presented with symptoms that could be related to CHD. For example, an episode of chest pain, even if ultimately diagnosed as non-cardiac, may offer an ideal opportunity to promote smoking cessation. Clinical assessment of suspected ACS is described in detail in Chapter 5.1. Chest pain is suggestive of MI if it radiates to either arm, both arms or shoulders; is described as burning, like indigestion, heavy, pressing or band-like; occurs on exertion; is associated with diaphoresis, nausea or vomiting; or is worse than previous angina or similar to previous MI. Chest pain is less likely to be MI if it is sharp, pleuritic, positional, reproduced by palpation, inframammary in location or not associated with exertion. Clinical assessment of cardiac pain is required to determine whether it is due to stable angina or ACS. Stable angina is characterized by pain that is predictable, precipitated by exertion, relieved promptly by rest or glyceryl trinitrate (GTN) and is not becoming more frequent or severe. Unstable angina is caused by a dynamic narrowing of the coronary artery and is characterized by pain that may be unpredictable, may occur at rest or minimal exertion, may not be immediately relieved by rest or GTN or may be increasing in frequency or severity. Patients with stable angina do not typically present to the ED. They are often used to their symptoms and will not seek medical help unless something unexpected happens. If a patient presents with apparently stable angina, the diagnosis should be considered carefully. It should be remembered that pain precipitated by exertion is known to be predictive of MI in ED patients. Clinical examination is generally unhelpful in making the diagnosis of ACS, which should be based on clinical history and investigations. However, clinical examination is essential to identify complications of ACS and to rule out differential diagnoses. Heart failure may be identified by poor peripheral circulation, tachycardia, pulmonary crepitations, elevated jugular venous pressure and a third heart sound on cardiac auscultation. The additional finding of hypotension may suggest cardiogenic shock. A systolic murmur raises the possibility of papillary muscle rupture or ventricular septal defect secondary to MI, although pre-existing aortic or mitral valve disease are much more common. Alternative diagnoses and their differentiation from ACS are described in Chapter 5.1. The most potentially serious alternative diagnoses are pulmonary embolus and aortic dissection. These should be considered in any patient with suspected ACS who is diaphoretic, tachycardic, tachypnoeic, hypotensive or reports associated neurological symptoms (transient or persistent) but does not have definite ECG features of ACS. The 12-lead ECG is the essential investigation to identify ACS requiring emergency reperfusion. It should be performed as soon as possible after arrival in any patient with any suspicion of ACS. Pre-hospital ECGs can be obtained by some emergency medical services and in some settings is used to prioritize patients and guide triage to high-dependency areas/cardiac catheter laboratories. The critical decision is to determine whether there is evidence of ST-elevation MI (STEMI) or MI with new bundle branch block. If there is any doubt, senior or specialist advice should be sought immediately. Repeat ECG recording may be helpful if a senior clinician feels there is insufficient certainty to allow for an immediate decision but a high suspicion of evolving MI persists. Identifying new bundle branch block presents a challenge, especially if previous medical records are not immediately available. In the past, the Sgarbossa criteria have been suggested as indicative of increased likelihood of MI. These criteria are ST elevation of 1 mm or more that is concordant with (in the same direction as) the QRS complex; ST depression of 1 mm or more in leads V1, V2 or V3 and ST-segment elevation of 5 mm or more that is discordant with (in the opposite direction to) the QRS complex. Recent research has questioned their utility, reporting low sensitivity for diagnosing MI. Other research has suggested that the presence of concordant ST changes is closely correlated with acute coronary occlusion but that the discordant criteria and left bundle branch block (LBBB) without Sgarbossa criteria are not. These findings have yet to be validated. In the absence of clear decision-making criteria, decisions regarding acute reperfusion will depend on specialist clinical judgement. Other ECG changes may be useful in diagnosing AMI and are described in Chapter 5.1 and Table 5.1.3. Q waves typically follow ST elevation but may appear as early as 4 hours after symptom onset. Their presence does not therefore preclude early reperfusion. Tall, upright T waves (‘hyperacute’ T waves) may be present in the very early stages of infarction. Deep (>3 mm) inverted T waves suggest a subendocardial MI which would be confirmed by biomarker elevation. Similarly, patients with significant (>1 mm) ST depression have an increased risk of adverse outcome and are likely to have a troponin rise. Unfortunately, despite an association with increased risk of adverse outcome, neither ST depression nor deep T-wave inversion is associated with benefit from acute reperfusion therapy. Other T-wave changes, such as small inversions (<3 mm), flat T waves and biphasic T waves, are common and non-specific. They may suggest ACS, but may also occur in patients with hypertension, patients who are hyperventilating and in the normal population. In addition to changes directly suggesting ACS, the ECG should be inspected for any concurrent pathology or evidence of complications. Cardiac rate and rhythm and P-wave presence and morphology should be evaluated for evidence of arrhythmia or heart block. Tall R waves or S waves suggest ventricular strain or hypertrophy that may contribute to or be a consequence of ACS. A subtle sign that can indicate ischaemia or ventricular dysfunction is poor anterior R-wave progression. Normally, R waves progressively increase in size across leads V1 to V4. Small R waves across these leads suggest pathology. Repeated 12-lead ECG recording or continuous ST-segment monitoring can help to identify transient or dynamic ECG changes. The development of significant (>1 mm) ST deviation provides clear evidence of ischaemia, identifies high-risk patients and may facilitate rapid identification of patients requiring reperfusion. T-wave changes, by contrast, are non-specific and often arise as a result of hyperventilation or changes in patient position during monitoring. The incidence of significant ST changes decreases and the incidence of false-positive T-wave changes increases in patients with a lower likelihood of significant ACS. Therefore, repeated ECG recording and ST-segment monitoring should be reserved for high-risk patients. A normal or non-diagnostic ECG does not rule out ACS or necessarily stratify the patient to a very low-risk group. In fact, the majority of patients admitted with ACS do not have diagnostic ECG changes. Serial ECG recordings and ST-segment monitoring do not substantially increase the negative predictive value of the ECG or provide very useful prognostic data. Negative ECG recording therefore has limited value. Biochemical markers are discussed in detail in Chapter 5.1. Their role is to identify patients with probable ACS and to rule out ACS if negative. However, it should be remembered that a negative cardiac marker, even if highly sensitive and performed at an optimal time after the worst symptoms, does not rule out CHD. Patients with negative markers require risk stratification and further cardiac testing if CHD is considered a likely diagnosis, although further cardiac testing does not necessarily have to be undertaken at the initial hospital attendance. Biochemical markers (particularly troponin) have a valuable prognostic role. Any patient with an elevated troponin is at increased risk of adverse outcome and has the potential to benefit from hospital admission, although the prognostic importance of small elevations of a high sensitivity assay is uncertain. If ACS is the likely cause of a troponin elevation, then the patient should be admitted under the care of a cardiologist. As a general rule, the higher the troponin level the greater the risk of adverse outcome. Patients with minor troponin elevations may be managed conservatively and possibly without ECG monitoring. A recent paper has reported the rate of significant ventricular arrhythmia in patients with troponin rise without ischaemic ECG changes as 0% (95% CI 0–2.3%). Those with substantial troponin elevations should be managed in a coronary care unit and considered for early percutaneous coronary intervention, even if they have no significant ECG changes. The term ACS covers a spectrum of disorders, including unstable angina, non-ST-elevation MI (NSTEMI) and STEMI. The diagnostic definition of MI has been a matter of intense debate in recent years and a consensus has gradually emerged. In contrast, the challenge of defining a diagnosis of ACS per se has been largely overlooked. The original World Health Organization (WHO) diagnosis of MI is outlined in Table 5.2.1. It required an elevation of creatine kinase to more than twice the upper limit of the normal range. With the development of troponins, it became apparent that this definition failed to include a substantial number of patients with prognostically significant myocardial damage, as evidenced by a troponin rise. Therefore, the American Heart Association and European Society of Cardiology (AHA/ESC) developed a new definition of MI, outlined in Table 5.2.2, which required a rise in serum troponin above the 99th percentile of the values for a reference control group. Table 5.2.1 WHO criteria for definite acute MI (1970) Table 5.2.2 The AHA/ESC criteria for MI (2012) The AHA/ESC definition has been widely adopted, despite a number of concerns and criticisms. Patients with ACS who fulfil this definition have a higher risk of adverse outcome than those who do not. However, patients with MI according to the AHA/ESC criteria alone have a lower risk of adverse outcome than those who fulfil both the AHA/ESC and WHO criteria. This has led to problems in maintaining consistent care over time and some experts have suggested identifying a threshold level for troponin (e.g. troponin T>1 ng/mL) above which clinically important MI should be diagnosed. This controversy is unlikely to be resolved in the near future, particularly as newer and more sensitive biochemical markers are developed. However, the most important issue to recognize is that any detectable troponin is associated with a potentially increased risk of adverse outcome and, the higher the troponin level, the higher that risk. MI can be usefully defined as STEMI or NSTEMI on the basis of the ECG. If there is evidence of significant ST elevation on any ECG (>2 mm in two consecutive chest leads, or>1 mm in two consecutive limb leads) then the patient has STEMI. These patients are likely to benefit from early reperfusion therapy. Patients without these changes but with evidence of myonecrosis based on cardiac markers are defined as having NSTEMI and do not benefit from reperfusion with thrombolytics, although PCI may be beneficial. NSTEMI and ACS without criteria for MI may be categorized together as non-ST-elevation ACS. AHA/ECS have introduced a classification system for MI based on underlying pathophyisological mechanisms. Of most relevance to emergency medicine practice are type 1 and type 2 MI. Type 1 MI is defined as spontaneous MI related to atherosclerotic plaque rupture, ulceration, fissuring, erosion or dissection with resulting intraluminal thrombus in one or more of the coronary arteries leading to decreased myocardial blood flow or distal platelet emboli with ensuing myocyte necrosis. Type 2 MI is defined as instances of myocardial injury with necrosis where a condition other than coronary artery disease (CAD) contributes to an imbalance between myocardial oxygen supply and/or demand, e.g. coronary endothelial dysfunction, coronary artery spasm, coronary embolism, tachy-/brady-arrhythmias, anaemia, respiratory failure, hypotension and hypertension with or without left ventricular hypertrophy (LVH). Some treatments are indicated for all ACS, whereas others have specific application to STEMI, NSTEMI and other ACS. Glyceryl trinitrate (GTN) and intravenous (IV) morphine are the analgesic agents of choice. Sublingual GTN may be appropriate if pain is mild to moderate, but severe pain usually requires titrated IV morphine. Doses of up to 20 mg, in small increments, are sometimes required. If IV morphine fails to control pain and the clinical condition is suitable, IV GTN by infusion at a rate titrated to effect (20–200 μg/min) is indicated. If this is insufficient to control pain and the patient is tachycardic, control of rate with small increments of IV β-blocker may be beneficial. It is important to note that ongoing severe pain, particularly in the absence of ECG changes, should raise concerns about an alternative diagnosis, such as aortic dissection. Aspirin 300 mg should be administered unless already given (e.g. by emergency services or general practitioner) or contraindicated. The principal contraindication to aspirin is known allergy. A previous history of gastritis or indigestion is not a contraindication to the use of aspirin in ACS. Recent analyses have raised questions about the role of routine oxygen therapy in the treatment for ACS. There is also a lack of evidence of benefit. Until further research clarifies the risk–benefit of supplemental oxygen therapy, its routine use is not recommended. Oxygen therapy is indicated for patients with hypoxia (oxygen saturation<93%) and those with evidence of shock to correct tissue hypoxia. Patients with STEMI who present within 12 hours of symptom onset should have a reperfusion strategy implemented emergently. Reperfusion can be obtained by fibrinolytic therapy, PCI, or rarely, with emergency coronary artery bypass grafting. The choice of reperfusion therapy will depend on time from symptom onset, availability of PCI, delay to fibrinolysis, contraindications to fibrinolysis, location and size of the infarct and the presence or absence of cardiogenic shock. PCI is the best available treatment if provided promptly. It is generally accepted that a delay of 90 minutes between presentation and balloon inflation is the maximum desirable. If this is not possible, fibrinolysis should be used. For patients presenting very early (symptom duration less than 1 hour), fibrinolytic therapy is highly effective, so the maximum tolerable delay to PCI is 1 hour from presentation. For patients aged less than 75 years with cardiogenic shock, PCI markedly improves outcomes. Fibrinolytic agents include streptokinase and tissue fibrin-specific agents, such as alteplase and tenectaplase. Available evidence suggests that fibrin-specific agents reduce mortality compared to streptokinase, despite an increased risk of intracranial bleeding. Note that streptokinase should not be given to patients who have been previously exposed to it (more than 5 days ago) due to antibody formation. There is also some evidence that it may be less effective in populations with high levels of exposure to streptococcal skin infections, such as Aboriginal and Torres Strait Islander peoples. Contraindications to fibrinolytic therapy are shown in Table 5.2.3. All patients receiving fibrinolyic therapy should be transferred to a PCI-capable centre for further assessment and treatment. In cases of failed fibrinolyisis or if there is evidence of re-occlusion/re-infarction (e.g. recurrent ST elevation), this transfer should be emergent. Table 5.2.3 Contraindications to fibrinolytic therapy in STEMI (ECS, 2011) Pre-hospital fibrinolysis should be considered when delay to PCI exceeds 90 minutes and transfer times to a fibrinolysis-capable facility exceed 30 minutes. The evolution of primary PCI has resulted in some systems identifying STEMI prior to hospital arrival and bypassing the ED, instead taking the patient directly to the catheterization laboratory. Even in these systems, it is important that emergency physicians remain knowledgeable and skilled in the diagnosis and management of STEMI, as a substantial proportion of cases self-present to the ED and pre-hospital diagnosis can sometimes be inaccurate. Patients undergoing primary PCI for reperfusion for STEMI should receive an antiplatelet agent. The choice of agent will depend on balancing the risk of recurrent ischaemic events and bleeding risk in individual patients. If clopidogrel is used it should be given as a high-dose clopidogrel regimen (600 mg oral bolus and 150 mg daily for 7 days, then 75 mg/day for at least 12 months). Potent oral antiplatelet agents (prasugrel and ticagrelor) are alternatives to clopidogrel for subgroups at high risk of recurrent ischaemic events (e.g. those with diabetes, stent thrombosis, recurrent events on clopidogrel or a high burden of disease on angiography). Prasugrel is contraindicated in patients with prior stroke/transient ischaemic attack. Neither prasugrel or ticagrelor should be used in patients with a previous haemorrhagic stroke or in patients with a moderate-to-severe liver disease. For patients undergoing fibrinolysis, 300 mg clopidogrel is recommended. Antithrombin therapy should be used in conjunction with PCI and fibrin-specific fibrinolytic agents. The use of antithrombin therapy with Streptokinase is optional. Low molecular weight heparin (e.g. enoxaparin) or unfractionated heparin can be used. There are data suggesting that enoxaparin may provide benefit over unfractionated heparin and dosing and administration are easier leading to recommendations listing it as the preferred agent. If unfractionated heparin is used it should be administered according to local dosing and monitoring guidelines taking into account whether there is concomitant use of glycoprotein IIb/IIIa inhibitors (GP inhibitors) and whether PCI or fibrinolysis was the reperfusion strategy used. Bivalirudin (a direct thrombin inhibitor) has shown similar efficacy to the heparins with less bleeding. Its place in the management of ACS is evolving. The role of GP inhibitors is evolving and data are conflicting and complex. Current recommendations are that the decision to use GP inhibitors is a specialist cardiologist decision and that there is no benefit in initiating it before the catheterization laboratory. An exception is that use before the catheterization laboratory may be considered in high-risk patients undergoing transfer for primary PCI in consultation with the receiving cardiologist. Abciximab is the preferred agent. In addition to aspirin, patients should receive P2Y12 receptor inhibitor agents, e.g. clopidogrel 300 mg loading dose and 75 mg/day. This should be withheld if emergency coronary bypass surgery is planned. There are limited data regarding newer antiplatelet agents (prasugrel and tricagrelor) in non-STEMI. In particular, data regarding prasugrel are limited to patients undergoing PCI. Both these agents have increased bleeding risk compared to clopidogrel. The decision to use them in preference to clopidogrel will require careful balancing of the bleeding risk and the risk of recurrent ischaemic events and is best left to the treating cardiologist. Subcutaneous low molecular weight or unfractionated heparin should be given until angiography or for 48–72 hours. The dose of low molecular weight heparin should be reduced if there is renal impairment. The dose of heparin is as above. Current evidence supports selective use of GP inhibitors. Indications may include ongoing ischaemia despite antiplatelet and antithrombin therapy, diabetes and troponin elevation. Initiation of a β-blocker is recommended unless contraindicated. Patients with NSTEMI should have early coronary angiography (ideally within 48 hours), unless they have severe co-morbidities. Disposition depends on the type of ACS. Patients with STEMI and NSTEMI require admission to hospital for further care. Those with STEMI should be admitted to a monitored bed in a cardiac care unit because of the small but significant risk of life-threatening arrhythmia. It has been the practice also to admit patients with NSTEMI to monitored beds, but this is being challenged on the basis that there are subgroups within this classification at very low risk of adverse events. Patients with ACS without ECG changes or cardiac marker elevations require a period of assessment in the ED/chest pain unit and disposition will depend on the risk identified during that process (see Chapter 5.1). Most MIs are accompanied by some degree of left ventricular failure, which may range in severity from asymptomatic to pulmonary oedema or cardiogenic shock. Mortality depends in part on the degree of left ventricular failure, with cardiogenic shock having a reported mortality of approximately 80%. Management includes maintaining adequate oxygenation, correcting electrolyte imbalances and optimizing ventricular filling pressures. Patients with pulmonary oedema may require non-invasive ventilatory support (see Chapter 5.3), but this need not be routine as most cases respond to medical therapy. If there is hypotension or other evidence of inadequate perfusion in the presence of adequate intravascular volume, inotropes should be initiated early and aggressively. PCI has been shown markedly to improve outcome for patients with STEMI accompanied by cardiogenic shock. Left ventricular assist devices may bridge to recovery, cardiac surgery or transplantation in selected patients. Thrombus can form on areas of hypokinetic myocardium due to relative stasis and the prothrombotic effects of local inflammatory changes. It is more common with large anterior infarctions with left ventricular aneurysm formation, where the incidence has been reported to be up to 10%. Echocardiography is used to confirm the presence of thrombus. Systemic anticoagulation is required to prevent embolic complications. Mechanical defects may include:
Cardiovascular Emergencies
5.1 Chest pain
Introduction
Epidemiology
Differential diagnosis
Musculoskeletal
Muscular strain
Epidemic myalgia
Tietze’s syndrome
Cardiac
Myocardial infarction
Unstable angina
Stable angina
Pericardial
Pneumomediastinum
Pericarditis
Gastro-oesophageal
Gastro-oesophageal reflux
Oesophageal spasm
Psychological
Anxiety/panic attacks
Hyperventilation
Cardiac neurosis
Pleuritic
Pulmonary embolus
Pneumothorax
Pleurisy
Pneumonia
Neurological
Cervical/thoracic nerve root compression
Herpes zoster
Abdominal
Peptic ulcer
Biliary colic/cholecystitis
Pancreatits
Mixed
Aortic dissection
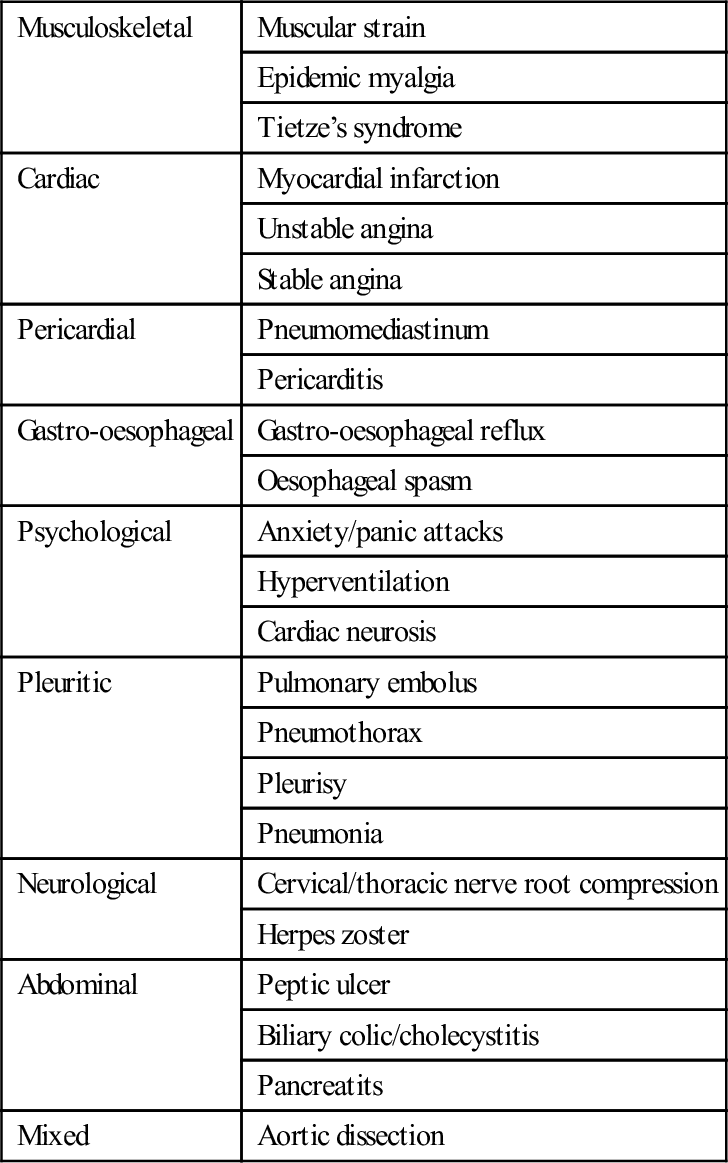
Clinical features
Useful for ruling in myocardial infarction
Radiation to the right arm or shoulder
4.7
Radiation to both arms or shoulders
4.1
Described as burning or like indigestion
2.8
Association with exertion
2.4
Radiation to left arm
2.3
Associated with diaphoresis
2.0
Associated with nausea or vomiting
1.9
Worse than previous angina or similar to previous myocardial infarction
1.8
Described as pressure
1.3
Useful for ruling out myocardial infarction
Described as pleuritic
0.2
Described as positional
0.3
Described as sharp
0.3
Reproducible by palpation
0.3
Inframammary location
0.8
Not associated with exertion
0.8
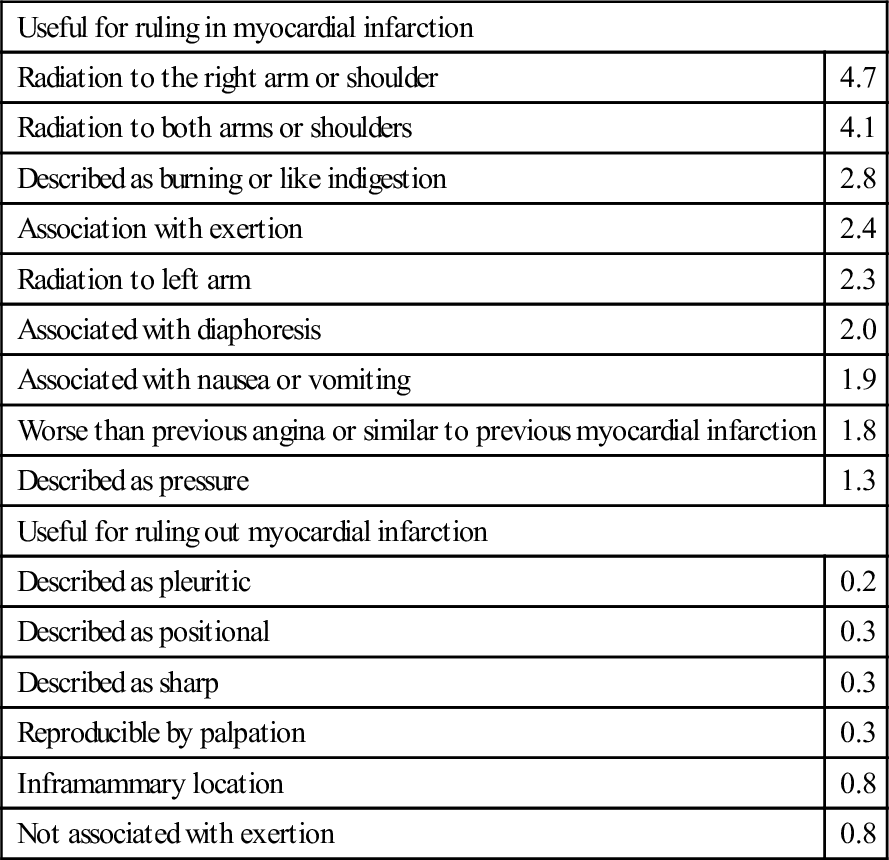
Clinical investigations
New ST elevation>1 mm
5.7–53.9
New Q wave
5.3–24.8
Any ST-segment elevation
11.2
New conduction defect
6.3
New ST-segment depression
3.0–5.2
Any Q wave
3.9
Any ST-segment depression
3.2
T-wave peaking and/or inversion>1 mm
3.1
New T-wave inversion
2.4–2.8
Any conduction defect
2.7
Biomarker
Sensitivity (%)
Specificity (%)
Troponin I*
77
93
Troponin T*
80
91
Roche high sensitivity troponin T
96
72
ADVIA Centaur Ultra high sensitivity troponin I
86
89
Abbot Architect high sensitivity troponin I
83
95
Quantitative heart-type fatty acid binding protein
81
80
Qualitative heart-type fatty acid binding protein
68
92
Myoglobin
62
83
Ischaemia modified albumen
77
39
Treatment
Prognosis
Likely developments over the next 5–10 years
5.2 Acute coronary syndromes
Introduction
Aetiology, pathogenesis and pathology
Epidemiology
Prevention
Clinical features
Differential diagnosis
Clinical investigations
Criteria for diagnosis
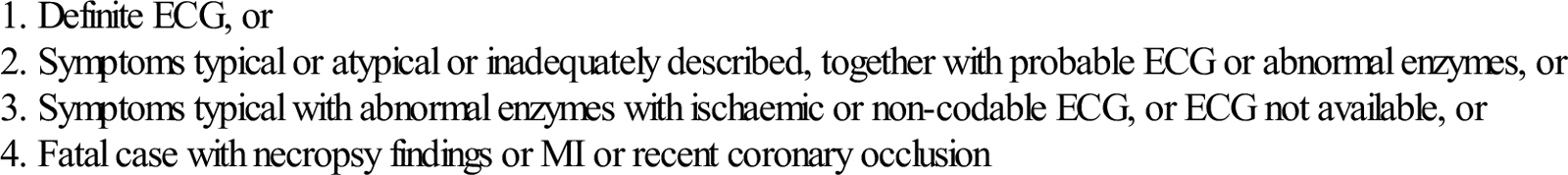
Typical rise and fall of biochemical markers of myocardial necrosis with at least one of the following:

Treatment
Treatments for all ACS
Analgesia
Aspirin
Oxygen
STEMI
Reperfusion

P2Y12 receptor inhibitors
Antithrombin therapy
Glycoprotein IIb/IIIa inhibitors
Non-STEMI
P2Y12 receptor inhibitors
Antithrombin therapy
Glycoprotein IIb/IIIa inhibitors
β-Blockers
Invasive management
Disposition
Complications
Arrhythmias and conduction disturbances (see Chapter 5.4)
Pericarditis (see Chapter 5.6)
Acute left ventricular failure and cardiogenic shock
Thromboembolism
Mechanical defects
 ventricular aneurysm formation with the attendant risk of thrombus formation and embolization
ventricular aneurysm formation with the attendant risk of thrombus formation and embolization
 acute mitral insufficiency secondary to papillary muscle dysfunction/rupture
acute mitral insufficiency secondary to papillary muscle dysfunction/rupture
 cardiac rupture, which may present as sudden death or acute pericardial tamponade.
cardiac rupture, which may present as sudden death or acute pericardial tamponade.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree