Edited by Mark Little Geoffrey Isbister Australia has a number of venomous snakes with some of the most potent venoms in the world. All the medically important snakes are elapids (front-fanged), although bites rarely occur from colubrids and non-venomous snakes. New Zealand has no snakes of medical importance. The risk of significant coagulopathy and uncommonly death, even after apparently trivial contact with Australian snakes, remains and must be appreciated by healthcare workers [1]. It is thought that approximately 3000 suspected snakebites occur annually in Australia, but this figure is difficult to estimate and depends on how many suspected bites, non-venomous bites and non-envenomed cases are included. The number of envenomed cases is far less and probably in the order of 100–200 each year; the majority of which occur in rural and regional areas. Snakebite deaths continue to occur (about 1–5 per year) and are usually a result of early cardiac arrest in brown snakebites or major haemorrhage in coagulopathic patients [1]. The commonest clinical manifestation is coagulopathy which occurs in about three-quarters of envenomed cases, the majority in brown snake bites [2]. Neurotoxicity and myotoxicity are now uncommon and mechanical ventilation is rarely required for treatment [2]. The types of snakes causing major envenoming differ across Australia. Bites in snake handlers remain an important problem with about 10% of all bites being in snake handlers. However, they are almost all bites from Australian snakes, albeit the more uncommon and interesting snakes and exotic snakebite is very rare [3]. Although snake handlers often want to avoid antivenom, they should be treated like anyone else because there is little evidence to support they are at higher risk of antivenom reactions. Snake handlers and people working with snake venoms can develop systemic hypersensitivity reactions to venom itself, so venom anaphylaxis must be a differential diagnosis in these patients [3]. Most snakebites are preventable and result from snake handling or interference with snakes in the wild, sometimes in the setting of alcohol consumption. Ideally, snakes should be left alone and those working with or keeping snakes should have appropriate training and licences. Simple precautions, such as wearing thick long pants and boots when walking in the bush or when working with snakes, can prevent most bites due to the short length of Australian elapid fangs. Snake handlers should carry and maintain first-aid kits that include at least four broad elastic bandages (15 cm; e.g. Ace) and have practised applying the bandage. If exotic snakes are being held, including Australasian snakes out of their geographical distribution, appropriate antivenoms should be available. Systemic envenoming results when venom is injected subcutaneously and reaches the systemic circulation. Whether or not a snakebite results in systemic envenoming depends on a number of factors including fang length, average venom yield of the snake, effectiveness of the bite and bite site. Recent studies have suggested that only a small amount of the injected venom actually reaches the systemic circulation [1,4]. Most snakebites do not result in envenoming because either insufficient venom reaches the systemic circulation or the snake is non-venomous. Envenoming is characterized by local and systemic effects, although Australasian elapids rarely cause major local effects, such as necrosis and local haemorrhage. The clinical features of envenoming depend on the particular toxins present in each snake’s venom but non-specific systemic symptoms (nausea, vomiting, headache, abdominal pain, diarrhoea and diaphoresis) occur in many cases. The major clinical syndromes are coagulopathy, neurotoxicity, myotoxicity and acute kidney injury [2]. Severe envenoming can result in early collapse associated with dizziness, loss of consciousness, apnoea and hypotension [1]. In the majority of cases, there is spontaneous recovery over 5–15 min but, in some case, this does not occur and multiorgan failure and death ensue if resuscitation is delayed [1]. The medically important Australian snakes and their associated clinical effects are listed in Table 30.1.1. Table 30.1.1 1VICC: venom-induced consumption coagulopathy; 4Polyvalent or tiger snake antivenom cannot be used for sea snake envenoming. This is the commonest and most important clinical effect in Australian snake envenoming. Venom-induced consumption coagulopathy (VICC) results from a prothrombin activator in the snake venom converting prothrombin (Factor II) to thrombin which leads to consumption of Factors V, VIII and fibrinogen, associated with a massive increase in fibrinogen degradation products [5]. Most dangerous Australian snakes contain such a prothrombin activator including brown snakes, snakes in the tiger snake group and taipans [5]. VICC develops rapidly within 15–60 min and the onset may coincide with the initial collapse seen with major envenoming by brown snakes and taipans [1]. Recovery usually takes 12–18 h [5]. Anticoagulant coagulopathy occurs in black snake envenoming, including mulga and red-bellied black snakes and is characterized by an abnormal activated partial thromboplastin time (aPTT) [6,7]. It is unlikely to result in haemorrhage and of itself is rarely of clinical importance. However, anticoagulant coagulopathy is a useful marker of envenoming and is rapidly reversed with antivenom [6]. Paralysis is a classic effect of snakebite and is due to mainly presynaptic neurotoxins that occur in almost all Australian elapids. Presynaptic neurotoxins disrupt neurotransmitter release from the terminal axon and are associated with cellular damage. This type of neurotoxicity does not respond to antivenom treatment and may take days to weeks to resolve in severe cases. Neurotoxic envenoming manifests as a progressive descending flaccid paralysis. The first sign is usually ptosis followed by facial and bulbar involvement and progressing to paralysis of the extraocular muscles, respiratory muscles and peripheral weakness in severe cases. Some Australian snakes contain myotoxins that cause damage to skeletal muscles resulting in local and/or generalized muscle pain, tenderness and weakness, associated with a rapidly rising creatine kinase and myoglobinuria. In rare severe cases, secondary renal impairment can occur. Renal impairment or acute kidney injury can occur in association with thrombotic microangiopathy, secondary to severe myolysis or, more rarely and to a minor degree, in isolation with brown snake envenoming. Thrombotic microangiopathy occurs in snakebites associated with VICC and is characterized by severe thrombocytopaenia worse 3–4 days after the bite, acute renal failure that may last 2–8 weeks and require dialysis and microangiopathic haemolytic anaemia [8]. It is most common with brown snake envenoming, but also reported with all snakes that cause VICC. Local effects vary from minimal effects with brown snakebites to local pain, swelling and, occasionally, tissue injury following black and tiger snakebites. Most fatalities occur within hours of the bite from initial cardiac arrest and multiorgan failure [1]. Delayed deaths are now uncommon and mainly due to major haemorrhage from VICC in brown snake, tiger snake group or taipan envenoming. Respiratory failure from neurotoxicity remains a problem in Papua New Guinea where there continue to be large numbers of cases, mainly taipan bites, and a shortage of both antivenom and resources for mechanical ventilation. Australian snake venoms appear to be absorbed via the lymphatic system so absorption is likely to be increased by movement and exercise. The aim of first aid is to minimize movement of venom to the systemic circulation. This is achieved by a pressure bandage (elastic bandage, such as ACE) being applied over the bite site and then covering the whole limb with a similar pressure to that used for a limb sprain. The bitten limb must be immobilized as well as the whole patient or the first aid is ineffective. Immobilization consists of splinting and complete prevention of movement or exercise of the bitten part. It has been shown that movement of all limbs, not just the affected one, needs to be minimized for optimal effect [9]. Transport should be brought to the patient and walking must be avoided. Pressure bandaging is clearly impractical for bites that are not on the limbs but direct pressure with a pad and immobilization may be useful. First aid must eventually be removed but this should take place in a resuscitation area of a facility with the means definitively to treat envenoming. The first aid is removed when: Figure 30.1.1 provides a simple approach to the management of suspected and envenomed snakebite patients. The patient is managed in an area with full resuscitation facilities. Assessment and management proceed simultaneously. The airway, breathing and circulation are assessed and stabilized. The majority of patients are not critically unwell and can have a focused neurological examination for early signs of paralysis (e.g. ptosis, drooling), examination of draining lymph nodes and general examination for signs of bleeding (oozing from the bite site, gum bleeds). Intravenous access should be established and intravenous fluids commenced. Two major diagnostic and risk assessment issues exist for snakebite: The majority of patients are not envenomed, but all patients must initially be assessed as if they are potentially envenomed. Asymptomatic patients, particularly those seen early after a brown snakebite, may still be severely envenomed with VICC. The diagnosis of envenoming is made on history, examination and the clinical investigations listed below. Although systemic envenoming can be ambiguous in patients with mild envenoming, the following definitions are useful for determining whether patients require antivenom: Table 30.1.2 provides a list of relative and absolute contraindications for antivenom which can be discussed with a clinical toxicologist if there is any doubt. If there is no evidence of envenoming after clinical assessment and initial laboratory testing, the first-aid bandage can be removed. The patient requires ongoing close observation including repeated investigations 1 h after bandage removal and at 6 and 12 h after the bite (see Fig. 30.1.1). Table 30.1.2 Absolute and relative indications for antivenom Absolute indications History of sudden collapse, cardiac arrest or seizure An abnormal INR Evidence of paralysis with ptosis and/or ophthalmoplegia being the earliest signs Relative indications: (suggest consultation with clinical toxicologist) Systemic symptoms (vomiting, headache, abdominal pain) Abnormal aPTT Creatinine kinase>1000 U/L Leucocytosis/lymphopaenia If the patient is envenomed, then management must proceed with antivenom. A small number of patients present in extremis, usually following collapse and in cardiac arrest and should have antivenom administered immediately as part of advanced life support. The next step is to determine the snake group responsible for envenoming in order to allow the administration of the appropriate monovalent antivenom. This is done taking into account: In the majority of cases, a combination of these two factors allows determination of the correct monovalent snake antivenom required. In some cases, an expert may be available to identify the snake. If the snake type cannot be determined based on geography and clinical syndrome, a snake venom detection kit (SVDK) may assist in identifying the snake. However, the results of an SVDK cannot be used in isolation from the geography, expert snake identification or clinical syndrome. If it is unclear which snake is involved then one vial of polyvalent antivenom should be administered or two vials of monovalent in regions (e.g. Victoria) where this will cover all medically important snakes – most commonly brown and tiger snake antivenoms. In Tasmania, only tiger snake antivenom is required. Snake antivenom should be administered by the intravenous route after being diluted 1 in 10 with normal saline and administered over 15 min. In patients with cardiac arrest or life-threatening effects, undiluted antivenom may be administered as a slow intravenous bolus. The dose of antivenom is one vial for all Australian snakes and the dose for children is the same as adults. Recovery is determined by the reversibility of effects and the time it takes for recovery once venom is neutralized. Repeat doses of antivenom are never required. Although there has been controversy over the dose of antivenom, recent studies have demonstrated that previously recommended large doses are not required [1,10]. Premedication for snake antivenom administration has previously been controversial but is no longer recommended in Australia. A recent randomized controlled trial has suggested that adrenaline is an effective premedication for snake antivenom [11], but this is more appropriate in resource poor settings where the risk of reactions is higher. Systemic hypersensitivity reactions occur in about one-fifth of antivenom administrations in Australia, but are only severe (mainly hypotension) in less than 5% of administrations [2,3]. Reactions are more common with tiger snake antivenom and polyvalent antivenom compared to brown snake antivenom [2,3]. Antivenom should always be administered in a critical care area with readily available adrenaline, intravenous fluids and resuscitation equipment. The frequency of delayed-type reactions to antivenom or serum sickness is probably higher than acute reactions and likely to depend on the amount of horse protein administered. All patients given antivenom should be warned of serum sickness. There is no evidence for the prophylactic use of a course of oral steroids but they should be used for treatment in patients who present with serum sickness (prednisolone 50 mg/day for 5–7 days). Tetanus prophylaxis should be given as appropriate but local wound care is rarely required with Australasian snakes due to minimal local effects. A recent randomized controlled trial has shown that the use of fresh frozen plasma (FFP) appears to speed the recovery of VICC [12], but whether the decreased risk of bleeding is large enough to balance the risk of blood products remains unclear. The study also suggested that use of FFP within 6 h of the bite may be associated with a poor response to FFP. Until larger studies are undertaken, FFP should be reserved for patients with coagulopathy and active bleeding. Assessment of the potentially envenomed requires the following investigations to be performed, usually serially: Repeat laboratory testing, particularly coagulation studies, should not be used to determine if sufficient antivenom has been given because one vial is sufficient in all cases. Such serial testing should be used to determine when the patient has recovered and can be discharged. The SVDK is designed to confirm which major snake group is responsible and therefore which antivenom to give. It does not confirm or exclude envenoming and should only be included in the assessment of envenomed patients after considering geography and clinical/laboratory effects. It is best done by laboratory staff. In non-envenomed patients, the SVDK has a high false-positive rate, especially in the brown snake well and is problematic in regions where brown snakes are uncommon (e.g. Victoria) [10]. A positive SVDK on urine does not indicate systemic toxicity and, in asymptomatic patients with normal laboratory studies, it is a false-positive result. The test should not be done on blood. Patients with suspected snakebite but no evidence of envenoming 1 h after the removal of first aid may be admitted to an observation area. Blood tests including coagulation studies and a CK should be repeated at 1 h after first aid is removed, and 6 and 12 h post-bite and be observed for 12 h or overnight (see Fig. 30.1.1). Envenomed patients requiring ventilatory support should have continued management in ICU, but patients with coagulopathy only are commonly managed in ED observation wards. Julian White The snakebite chapter of this edition is targeted principally at the Australian snakebite experience, but snakebite is a global phenomenon, arguably with>2.5 million cases,>100000 deaths and>400000 amputations every year, so Australia accounts for only a tiny fraction of this impact. Exotic snakebite is a worldwide problem, with increasing seizures by customs of illegally imported snakes and seizures of illegal collections by authorities in countries. Some think that the trade in exotic animals is second only to the illegal trade of drugs and weapons. Exotic snakebite in Australia is either where an Australian snake species bites a person in a region where this snake is not usually found (e.g. pet taipan bites owner in Hobart), or where a snake, not native to Australia, bites someone in Australia. This chapter will focus on this second scenario. This topic is vast and beyond the scope of this chapter, but similar management principles may apply. Table 30.2.1 provides a list of selected genera/species, with distribution, clinical effects and major modes of treatment. Table 30.2.1 Selected exotic snakes; overview of clinical effects and management
Toxinology Emergencies
30.1 Snakebite
Introduction
Epidemiology
Prevention
Clinical features
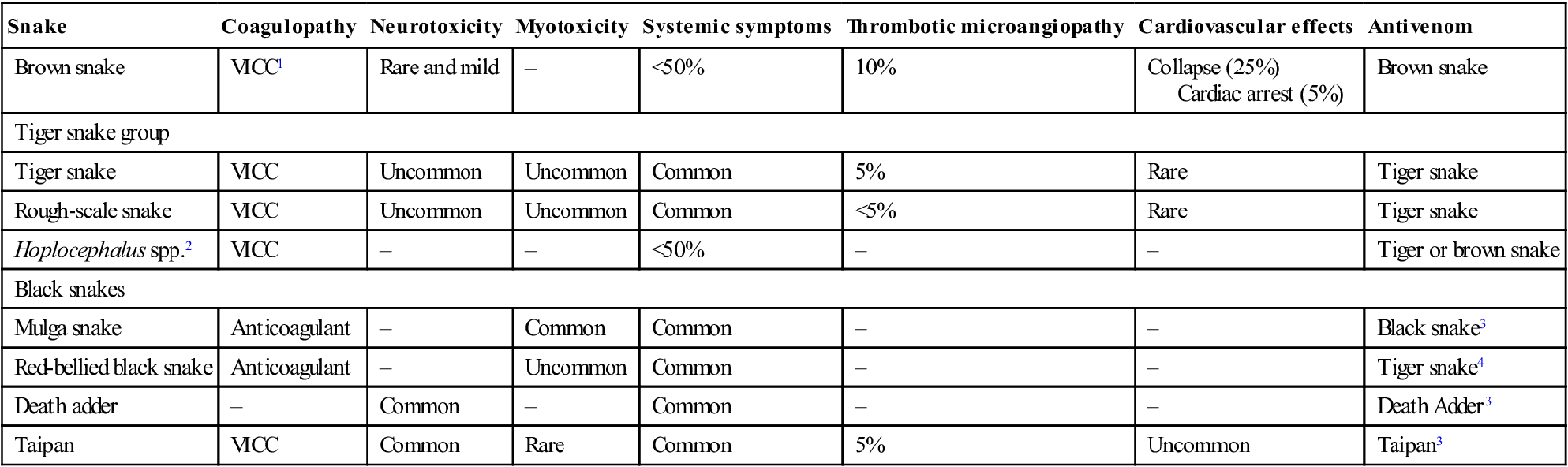
Snake
Coagulopathy
Neurotoxicity
Myotoxicity
Systemic symptoms
Thrombotic microangiopathy
Cardiovascular effects
Antivenom
Brown snake
VICC1
Rare and mild
–
<50%
10%
Collapse (25%)
Cardiac arrest (5%)
Brown snake
Tiger snake group
Tiger snake
VICC
Uncommon
Uncommon
Common
5%
Rare
Tiger snake
Rough-scale snake
VICC
Uncommon
Uncommon
Common
<5%
Rare
Tiger snake
Hoplocephalus spp.2
VICC
–
–
<50%
–
–
Tiger or brown snake
Black snakes
Mulga snake
Anticoagulant
–
Common
Common
–
–
Black snake3
Red-bellied black snake
Anticoagulant
–
Uncommon
Common
–
–
Tiger snake4
Death adder
–
Common
–
Common
–
–
Death Adder3
Taipan
VICC
Common
Rare
Common
5%
Uncommon
Taipan3
Coagulopathy
Venom-induced consumption coagulopathy (VICC)
Anticoagulant coagulopathy
Neurotoxicity
Myotoxicity
Renal toxicity
Local effects
Treatment
First aid
Initial assessment and treatment
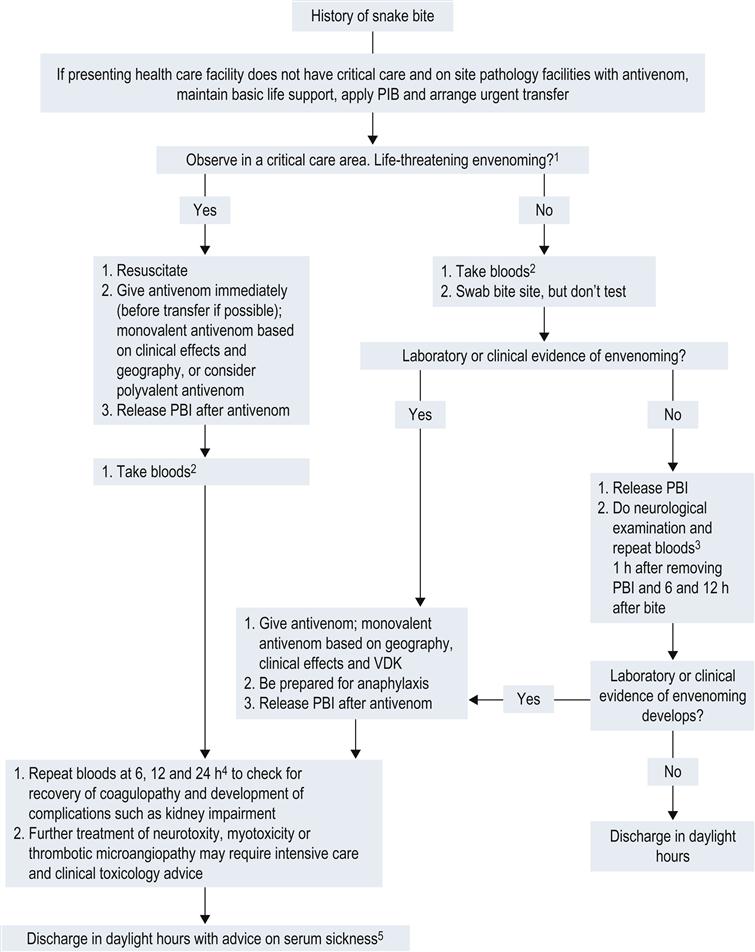
Further management
Administration of antivenom
Other treatments
Clinical investigations
 full blood count including a blood film looking for fragments, red cells and evidence of haemolysis
full blood count including a blood film looking for fragments, red cells and evidence of haemolysis
 urea, creatinine, electrolytes, creatine kinase (CK) and lactate dehydrogenase
urea, creatinine, electrolytes, creatine kinase (CK) and lactate dehydrogenase
 snake venom detection kit: a swab should be taken from the bite site
snake venom detection kit: a swab should be taken from the bite site
Snake venom detection kit
Disposition
30.2 Exotic snakebite
Introduction
Snake
Distribution†
Clinical effects‡
Treatment 
Family ‘Colubridae’#
Boomslang (Dispholidus typus)
SSaf
CC, NF, BH, HF
AV, BP, IV, NC
Bird/vine/twig snakes (Thelotornis spp.)
SSaf
CC, NF, BH, HF
BP, IV, NC
Keelback & yamakagashi (Rhabdophis spp.)
SEAs, EAs
CC, BH, HF
AV, IV, BP
Family Elapidae
PNG small eyed snake (Micropechis ikaheka)
PNG (New Guinea)
PU, M, AC, NF
AV, IV, NC, ST
Bolo (Ogmodon vitianus)
PNG (Fiji)
?LS
IV, ST
Bougainville coral snake (Parapistocalamas hedigeri)
PNG (Bougainville)
?LS
IV, ST
Solomons coral snake (Salomonelaps par)
PNG (Solomon Islands)
?LS
IV, ST
PNG forest snakes (Toxicocalamus spp.)
PNG (New Guinea)
?LS
IV, ST
Asian coral snakes (Calliophis spp.)
SEAs
PU, RF
IV, ST, AC
Asian spitting cobras (Naja spp.)
SEAs, EAs, Ind
PN, SO, LI, HF
AV, IV, LC, AC
Asian cobras (Naja spp.)
SEAs, EAs, Ind, As
PN, RF, LI (some)
AV, IV, LC, AC
King cobra (Ophiophagus hannah)
SEAs, Ind
PN, RF, LI, HF
AV, IV, LC, AC, ST
Kraits (Bungarus spp.)
SEAs, EAs, Ind
PP, PN, RF, M
AV, IV, ST
Desert black snake (Walterinnesia aegyptia)
ME, NtAf
PN, RF
ST, IV, ?AV1
Water cobras (Naja (ex Boulengerina) spp.)
SSAf
PN, RF
ST, IV
African spitting cobras (Naja spp.)
SSAf, NtAf, ME
SO, LI, PN, HF
AV, IV, LC
African cobras (Naja spp.)
SSAf, NtAf, ME
PN, RF, LI (some)
AV, IV, LC, ST, AC?
Mambas (Dendroaspis spp.)
SSAf
PD, RF, LI (some)
AV, IV, ST, LC
Rinkhals (Hemachatus haemachatus)
SSAf
LI, PN
AV, IV, LC, ST
African coral snakes (Aspidelaps spp.)
SSAf
PN, RF
IV, ST, AC?
African garter snakes (Elapsoidea spp.)
SSAf
LS
IV, ST
Tree cobras (Pseudohaje spp.)
SSAf
LS
IV, ST
Spotted harlequin snakes (Homoroselaps spp.)
SSAf
?LS
IV, ST
Burrowing cobra (Paranaja spp.)
SSAf
LS
IV, ST
American coral snakes (Micrurus, Leptomicrurus spp.)
NtAm, CeAm, StAm
PN, PP (some), M, RF
AV, IV, ST
US coral snake (Micruroides euryxanthus)
NTAm
PN, RF
IV, ST
Sea snakes (many species)
Indo-Pacific
PN, RF, M, NF
AV, IV, ST, AC, NC
Family Viperidae (Viperinae; old world, non-pit-vipers/adders)
Russell’s vipers (Daboia spp.)
SEAs, EAs, Ind
CC, BH, BD, BS, NF, HF, LI, PU, RF, M
AV, IV, ST, LC, NC, BP
Saw scaled vipers (Echis spp.)
Ind, WAs, ME, NtAf, SSAf
CC, BH, BD, NF, HF, LI
AV, IV, ST, LC, NC, BP
Horned vipers (Pseudocerastes spp.)
ME, WAs
LS, PU?
IV, ST
Horned vipers (Cerastes spp.)
ME, NtAf
LI, CC, BH, NF, HF
AV, IV, ST, LC, NC, BP
Puff & Gaboon adders (Bitis spp.)
SSAf, NtAf
LI, HF, BD
AV, IV, ST, LC
Berg adders (Bitis atropos, etc.)
SSAf
LI, HF, PU, RF
IV, ST, LC
Night adders (Causus spp.)
SSAf, NtAf
LS, PU
IV, ST, LC
Bush vipers (Atheris, Montatheris, Proatheris spp.)
SSAf
LS, CC, HF
AV2, IV, ST, LC, BP
McMahon’s viper (Eristocophis mcmahoni)
WAs, ME
LI, HF, PU?
IV, ST, LC
Barbour’s bush viper (Adenorhinos barbouri)
SSAf
LS
IV, ST
Fea’s viper (Azemiops feae)
EAs, SEAs, As
LS
IV, ST
European adders (Vipera, Macrovipera spp.)
NtAf, EU, ME, As
LI, CC, HF, BD, PU
AV, IV, ST, LC, BP
Family Viperidae (Crotalinae; pit vipers)
Copperhead, cottonmouth, cantils (Agkistrodon spp.)
NtAm, CeAm
LI, CC, HF, BD, NF
AV, IV, ST, LC
Jumping vipers (Atropoides spp.)
CeAm
LS, HF
IV, ST, LC
Lancehead vipers (Bothrops spp.)
StAm, CeAm
LI, HF, CC, BD, NF, LA, RF, DV (Caribbean spp.)
AV, IV, ST, LC, NC
Palm pit vipers (Bothriechis spp.)
CeAm
LI, HF, BD
AV, IV, ST, LC, NC
Malayan pit viper (Calloselasma rhodostoma)
SEAs
LI, HF, CC, BH, BD, NF
AV, IV, ST, LC, NC
Montane pit vipers (Cerriphidion spp.)
CeAm
LI, HF, BD
AV, IV, ST, LC, NC
North American rattlesnakes (Crotalus spp.)
NtAm
LI, HF, CC, BH, BD, NF, PP & RF (few spp.)
AV, IV, ST, LC, NC, BP
South American rattlesnakes (Crotalus spp.)
CeAm, StAm
CC, BH, M, PP, RF, NF
AV, IV, ST, NC
Hundred pace viper (Deinagkistrodon acutus)
EAs
LI, HF, BD, NF
AV, IV, ST, LC, NC
Mamushis, etc (Gloydius spp.)
EAs, SEAs
LI, HF, CC, BD, PU, RF, M, NF
AV, IV, ST, LC, NC
Hump nosed vipers (Hypnale spp.)
Ind
LI, HF, CC, BH, NF
IV, ST, LC, NC
Bushmaster (Lachesis spp.)
CeAm, StAm
LI, HF, CC, BH, BD
AV, IV, ST, LC
Horned pit viper (Ophryacus spp.)
CeAm
LI, HF
IV, ST, LC
Montane pit vipers (Porthidium spp.)
CeAm
LI, HF
IV, ST, LC
Habus (Protobothrops spp.)
EAs, Ind
LI, HF, CC, BH, BD
AV, IV, ST, LC, NC
Pygmy rattlesnakes (Sistrurus spp.)
NtAm
LI, HF, CC, BD
AV, IV, ST, LC
Green tree vipers (Trimeresurus spp. incorporating spp. variously assigned to the genera Ovophis, Crypteletrops, Popeia, Parias, Viridovipera, Himalayophis, Peltopelor)
SEAs, EAs, Ind
(varies significantly between species) LI, HF, CC, BH, BD, NF
AV, IV, ST, LC, NC, BP
Temple pit vipers (Tropidolaemus spp.)
SEAs, Ind
LI, HF
IV, ST, LC
Mount Mang pit viper (Protobothrops (ex Zhaoermia) mangshanensis)
EAs
LI, HF
IV, ST, LC ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


30. Toxinology Emergencies
Only gold members can continue reading. Log In or Register to continue