Pathophysiology
With the exception of distal urethra, the urinary tract is normally sterile. The resistance to UTI is influenced by exposure to uropathogenic bacteria, age, hormonal status, and urine flow (7,8). The insertion of an IUBC allows organisms to gain access to the bladder; the device induces an inflammation of the urethra, allowing bacteria to ascend into the bladder in the space between the urethral mucosa and the catheter. CAUTI usually follows formation of biofilm, which consists of adherent micro-organisms, their extracellular products, and host components deposited on both the internal and external catheter surface. The biofilm protects organisms from both antimicrobials and the host immune response (7,9). The ascending route of infection is predominant in women because of the short urethra and contamination with the anal flora. Intraluminal contamination is less frequent, and is related to reflux of pathogens from the drainage system into the bladder. This contamination occurs in case of failure of closed drainage or contamination of urine in the collection bag.
| TABLE 88.3.18 Catheter-associated Urinary Tract Infection (CAUTI): Patient Must Meet Criteria 1, 2, and 3 |
 |
Microbiology
The isolated pathogens among ICU patients with bacteriuria are essentially Escherichia coli, Pseudomonas aeruginosa, and Enterococcus species (1,10,11); polymicrobial infections represent only 5% to 12% (3,10) of cases. In the largest report investigating nosocomial infections in ICU patients, gram-negative bacteria are responsible for 70% of CAUTIs (1). During this period, resistance to antimicrobials increased, especially the rates of resistance to third-generation cephalosporins for E. coli (up to 60%) and for Klebsiella pneumoniae (68%) (Table 88.3.19) (1).
Risk Factors
In ICU patients, UTIs are associated with the presence of an IUBC. Among 10,755 patients in a trauma center, risk factors were female gender (59%) and age (>57 years old) (12). Accordingly to prior studies, female gender, length of ICU stay, prior use of antibiotics, severity score at admission, and duration of catheterization were independently associated with an increased risk of catheter-associated bacteriuria (13–15). These results emphasize that preventing or reducing the duration of catheterization is the most important intervention. In a tertiary care hospital, the initial indication for the placement of a urinary catheter was unjustified in 15% of patients and unclear in 28% of patients (14); as a result, the length of stay and cost of care were increased (15). In the medical ICU, an excessively prolonged use of urinary catheter for monitoring urine output resulted in 64% of the total unjustified patient days (16).
| TABLE 88.3.19 Percentage of Pathogens Associated with Nosocomial Urinary Tract Infections |
 |
Impact of CAUTIs in ICU
Although adverse consequences of asymptomatic UTIs are described during pregnancy or in nursing home (17,18), the real impact of ICU-acquired CAUTIs on outcome remains unclear. In a general hospital population, Platt et al. (19) showed that nosocomial UTIs were associated with a significant attributable mortality; the picture is probably different in a specific ICU population. Indeed, even after controlling for many risk factors, UTIs have a higher incidence in ICUs than on conventional wards (20), although urinary tract is the source of sepsis in only 10% to 14% of cases, far from that seen in the lung (Table 88.3.20) (1,4,21,22). The development of an ICU-acquired UTIs is associated with a prolonged ICU stay and crude rate of mortality (2,10); however, adequately powered studies demonstrate that UTIs are not dependent factors for mortality (2,23).
| TABLE 88.3.20 Rates of Sepsis According to Each Site | |||
 | |||
Urosepsis is defined as an inflammation of the upper urinary tract that causes seeding of the blood with bacteria, resulting in local and distant destruction of tissues. In a retrospective study, urinary tract was responsible for 21% of the health care–acquired bloodstream infection. The incidence was 1.4 urinary bloodstream per 10,000 patients days of IUBC, with an associated mortality rate at 15% (24). These results are similar in ICU population. In another retrospective study, a total of 105 CAUTIs were identified; only 6% resulted in positive bloodstream infections. The mortality rates of the patients were not influenced by the presence of CAUTI (5). Accordingly, in a prospective randomized study, 6 out of 60 patients with an IUBC and asymptomatic bacteriuria developed urosepsis (25). Risk factors of developing an urinary tract–related bloodstream infection were neutropenia, renal disease, male gender, insulin, and immunosuppressant therapy (26).
Finally, the CAUTIs result in a significant increase in cost. An episode of symptomatic nosocomial CAUTI in hospitalized patients was associated with an additional cost of US$749—1,007/admission (24,25). Among the nosocomial infections, UTIs had the lowest daily antibiotic cost per infected patient (27).
DIAGNOSTIC TOOLS IN THE ICU
The diagnosis of urosepsis should be entertained each time a patient has a febrile episode. Because of the prevalence of bacteriuria in patients with urinary catheters, some have advocated daily monitoring of urine in catheterized patients; however, routine daily bacteriologic monitoring of the urine from all catheterized patients is not an effective way to decrease the incidence of symptomatic, catheter-associated UTI and is not recommended (28)
Some clinical trials assess the effectiveness of urinary dipsticks (leukocyte esterase and nitrite) for screening patients instead of quantitative urine culture in ICU (Table 88.3.21) (29,30). Leukocyte esterase activity is an indicator of pyuria and urinary nitrite production an indicator of bacteriuria. In an older medical ICU study, it had been demonstrated that the urinary dipstick strategy was a rapid and cost-effective test with which to screen asymptomatic catheterized patients (29,31,32). This effectiveness was observed only for a positive quantitative urine culture level of 105 organisms/mL; in these cases, the urinary dipstick strategy decreased the cost of nosocomial infection diagnosis and the daily workload in the microbiology laboratory. The authors concluded that the urinary dipsticks were a cost-effective test for screening asymptomatic catheterized patients in a medical ICU (29). During a 2-year period, however, Coman et al. (30) did not show an impact of urinary dipstick on symptomatic CAUTI with fever or hypothermia. The Cochrane review concluded that the effectiveness of urinary dipstick remains unresolved due to the lack of good quality studies (33). The use of dipsticks instead of quantitative urine culture cannot be recommended for symptomatic CAUTIs in ICU patients. Guidelines recommend that asymptomatic bacteriuria or funguria should not be screened for in patients with IUBCs (34). Hence, in symptomatic patients, quantitative urine culture with Gram stain examination is recommended to obtain rapid identification of the pathogen.
| TABLE 88.3.21 Assessing Urinary Reagent Strips in the ICU | |||
 | |||
PREVENTION OF UTI IN THE ICU
Most measures, described below, are useful only in units with a restrictive policy of catheterization (Table 88.3.22).
Urinary Drainage System
For preventing infection, the maintenance of a closed, sterile drainage system is recommended (35); this was described for the first time in 1928 (36), and its benefit has been subsequently re-enforced. In a randomized study, a subgroup analysis, which considered patients not receiving an antibiotic treatment, showed a reduction in mortality in the group using the closed system (37). Historically, “open systems” were large, uncapped glass bottles. The drainage catheters were inserted into the glass bottles, often below the level of urine; urine was stagnant, and bacteria could easily grow and ascend through the drainage catheter. The introduction of closed drainage systems was an improvement, dramatically reducing the rate of bacteriuria. However, in the modern era, several studies failed to confirm the benefit of complex closed system compared with simple devices (38,39).
Two studies focused specifically on ICU patients (40,41), comparing a two-chamber drainage system with a complex closed drainage system. In a randomized and prospective trial, 311 patients requiring an IUBC for longer than 48 hours were assigned to the two-chamber drainage system group or to a complex closed drainage system group to compare the rates of bacteriuria. Rates of UTIs were 12.1 and 12.8 episodes/1,000 catheter days, respectively (p > 0.05) (40). The data extracted from the literature do not support the use of complex closed drainage systems in ICU patients in view of the increased cost (42).
| TABLE 88.3.22 Comparative Studies Performed in the ICU on the Prevention of Catheter-associated Urinary Tract Infection |
 |
For the management of drainage systems, owing to the lack of specific studies in ICU, guidelines may be viewed as recommendations at best. The following are reasonable suggestions: only persons who know the correct technique of aseptic insertion and maintenance of the catheter should handle catheters; hospital personnel should be given periodic in-service training stressing the correct techniques and potential complications of urinary catheterization; hand-washing should be done immediately before and after any manipulation of the catheter site or apparatus; if small volumes of fresh urine are needed for examination, the distal end of the catheter, or preferably the sampling port if present, should be cleansed with a disinfectant, and urine then aspirated with a sterile needle and syringe; larger volumes of urine for special analyses should be obtained aseptically from the drainage bag; unobstructed flow should be maintained; to achieve free flow of urine, (1) the catheter and the collecting tube should be kept from kinking; (2) the collecting bag should be emptied regularly using a separate collecting container for each patient (the draining spigot and nonsterile collecting container should never come in contact); (3) poorly functioning or obstructed catheters should not be irrigated in routine but replaced after reconsidering indication of urinary catheter; (4) collecting bags should always be kept below the level of the bladder; and (5) IUBCs should not be changed at arbitrary fixed intervals (34,43,44).
Type of Urethral Catheters
There is a vast literature stressing the efficacy of antiseptic impregnated catheters, including silver oxide or silver alloy, and antibiotic-impregnated catheters in hospitalized patients (45–47). In the largest RCT, Pickard et al. showed a nonclinically relevant benefit of using nitrofural-impregnated catheters to reduce CAUTI versus silver-coated or standard catheters (47). Diagnosis of CAUTI was supported by microbiologic culture and use of antibiotic, but the difference is probably not clinically relevant (48). A recent Cochrane meta-analysis compared the effectiveness of different indwelling urethral catheters in reducing risk of CAUTI for short-term catheterization (<14 days) (42); silver oxide and silvery alloy catheters were not associated with a reduction of CAUTI in short-term catheterized hospitalized adults. Catheters coated with a combination of minocycline and rifampin or nitrofurazone may be beneficial in reducing bacteriuria and symptomatic CAUTI in hospitalized males catheterized less than 2 weeks, but this requires further assessment (42). Comparison of nitrofurazone-coated and silver alloy–coated catheters results in a superiority of antibiotic-coated in reducing bacteriuria and symptomatic CAUTI, but the magnitude of reduction was low and hence may not be clinically relevant (42). However, those catheters are more expensive and are more likely to cause discomfort than standard catheters (42).
In ICU patients, a randomized, prospective, double-blind multicenter trial compared catheters coated with hydrogel and silver salts with classical urinary tract catheters (49). The cumulative incidence of UTIs associated with catheterization was 11.1% overall, 11.9% for the control group, and 10% for the coated catheter group. The odds ratio was 0.82 (95% confidence interval [CI]: 0.30 to 2.20) (49). In a prior blind prospective trial, standard latex IUBCs were switched for a hydrogel latex IUBC with a monolayer of silver metal applied to the inner and outer surfaces of the catheter. The adjusted CAUTI rates during the baseline and intervention periods were 8.1 and 4.9 infections/1,000 device days, respectively (p = 0.13) (47). With respect to long-term bladder drainage, a Cochrane meta-analysis showed that no eligible trials compared alternative routes of catheter insertion or catheter types (48).
Meatal Care
Twice daily cleansing with povidone–iodine solution and daily cleansing with soap and water have failed to reduce CAUTIs; thus, at this time, daily meatal care with either of these two regimens cannot be endorsed (34). A randomized, controlled, prospective clinical trial involving 696 hospitalized medical and surgical patients was undertaken to determine the effectiveness of 1% silver sulfadiazine cream applied twice daily to the urethral meatus in preventing transurethral catheter-associated bacteriuria. The overall incidence of bacteriuria was similar in both groups (p = 0.56) (42). In the absence of study performed in a specific ICU patient population, the expert opinion may be followed and daily soap cleansing seems to be an appropriated care (44).
There are no data available on the level of sterility required to insert the urinary catheter. Experts recommend that catheters should be inserted using aseptic technique and sterile equipment; gloves, drape, and sponges should be used for insertion. However, in a prospective study conducted in the operating room, 156 patients underwent preoperative urethral catheterization, randomly allocated to “sterile” or “clean/nonsterile” technique groups. There was no statistical difference between the two groups with respect to the incidence of UTI, but the cost differs considerably between the two groups (43). A Cochrane meta-analysis of 31 trials showed no evidence that the incidence of CAUTI is affected by the use of aseptic or clean technique for intermittent catheterization in long-term bladder management (50).
Bladder Irrigation and Antiseptic in the Drainage System
The objective of antibiotic irrigation is to clear the bacteria from the urinary tract. A randomized study compared 89 patients receiving a neomycin–polymyxin irrigant administered through closed urinary catheters to 98 patients not given irrigation. Eighteen of 98 (18%) of the patients not given irrigation became infected, as compared with 14 of 89 (16%) of those given irrigation, and the organisms from patients with irrigation were more resistant (51). Another study was conducted in urology patients, evaluating the effect of povidone–iodine bladder irrigation prior to catheter removal on subsequent bacteriuria. Of 264 patients, 138 received irrigation and 126 were controls. Urine cultures were positive in 22% in the control group and 18% in the study group (52). Thus, irrigation methods failed to demonstrate an efficacy in surgical patients and meta-analysis does not shows any benefit for long-term catheterization management (53). Experts do not recommend its use in ICU patients (34,44).
In ICU, the addition of antimicrobial agents in the drainage device has not been studied. The largest study investigating the effect of H2O2 insertion in the drainage device of 353 patients compared to 315 control patients failed to show a benefit in treated patients. It is noteworthy that 68% of these patients required an IUBC for hemodynamic monitoring, with antimicrobial therapy prescribed in 75% of patients, suggesting these results can apply to ICU patients (54). Experts recommend not using any kind of irrigation unless obstruction is anticipated, as might occur with bleeding after prostatic or bladder surgery (34,44).
Alternatives to the Urinary Catheter
For selected patients, other methods of urinary drainage such as condom catheter drainage, suprapubic catheterization, and intermittent urethral catheterization should be used as alternatives to an IUBC. While there are few data available in ICU to assess these alternative devices, there is evidence that suprapubic catheterization have advantages over indwelling catheters with respect to bacteriuria, recatheterization, and discomfort after abdominal or pelvic surgery (55–57). The use of condom linked to a collection bag has been evaluated in a study comparing two periods of 6 months, in which 167 patients were included. The occurrence of bacteriuria was significantly decreased for the period using the condoms (26.7% vs. 2.4%) (58). A recent study comparing microbiology reports from cultures collected from external versus indwelling catheters shows no difference in species that colonize, but did not analyze UTI incidence (59). A randomized trial of 75 males older than 40 years compared condom and indwelling catheters. Morbidity risk (bacteriuria, symptomatic UTI, or death) was higher in the catheterized group (hazard ratio = 4.84, 95% CI = 1.46 to 16.02; p = 0.02). Patients reported that condom catheters were more comfortable (p = 0.02) and less painful (p = 0.02) than indwelling catheters (60). The use of intermittent catheterization was also associated with a lower risk of bacteriuria than indwelling urethral catheter; such a procedure has not been systematically investigated in ICU patients (42,61,62).
Miscellaneous Measures
While there is a low risk of bacteremia during the urinary catheterization (63), the administration of prophylactic antimicrobial therapy at the time of catheterization leads to a reduction in bacteriuria and pyuria (64). The efficacy of antibiotic treatment has been assessed as optimal for catheterization lasting less than 14 days in perioperative and nonsurgical patients (64). However, the prophylactic use of antibiotic in ICU can be detrimental for the ecology in increasing the resistance of bacteria. This practice must, therefore, be discouraged in ICU. It is noteworthy that in most ICU studies 75% of patients with an indwelling catheter required antibiotics for different reasons (10).
Care bundles seem efficient to reduce CAUTI in conventional wards. A large survey compared American hospitals applying the “Keystone bladder bundle initiative” (65) with those not applying it (64). The compliant hospitals had better prevention of CAUTI related to improved assessment of initial indication, use of bladder scanner, removal reminder, and/or systematic stop orders (66,67). In the ICU, use of a daily checklist to systematically review invasive devices may increase compliance with recommendations for preventing nosocomial infection, but as of this writing, there is no reduction of CAUTI (68). Emerging work aiming at removing biofilm in order to prevent CAUTI by using low-energy ultrasound (69) or lytic bacteriophages (70) are underway; to date, they are not applicable to the bedside.
In conclusion, few preventive measures have demonstrated efficacy in reducing the rate of UTIs in the ICU. Additionally, the clinical significance of bacteriuria remains uncertain. Consequently, general measures with good adherence to hygiene procedures are more relevant than expensive devices to fight infections.
TREATMENT OF CAUTIS IN THE ICU
The management of CAUTI has not been evaluated in ICU patients. Several nonspecific measures, including hydration, have been advocated in the therapy of UTI. Adequate hydration would appear to be important although there is no evidence that it improves the effectiveness of an appropriate antimicrobial therapy (71). The management of complicated UTIs in the ICU may include mechanical intervention. Consequently, appropriate diagnostic tests and urologic consultation should be included in the algorithm of the management of these patients (Fig. 88.3.1).

FIGURE 88.3.1 Suggested algorithm for the management of urinary tract infections related to an indwelling catheter in the ICU. *Discuss the need for antipseudomonal coverage according to the duration of hospitalization, prior medical history, and local ecology. #Discuss if renal failure.
Management of Asymptomatic Bacteriuria
Expert opinion holds that asymptomatic catheter-associated bacteriuria does not require treatment or screening in the ICU (34,72). However, antimicrobial treatment may be considered for asymptomatic women with a CAUTI that persists 48 hours after catheter removal (73). In a specific ICU population, 60 patients with an IUBC for longer than 48 hours who developed an asymptomatic positive urine culture were randomized to receive either a 3-day course of antibiotics associated with the replacement of the indwelling urethral catheter 4 hours after first antibiotic administration or no antibiotics and no catheter replacement; six patients, equally distributed in the two groups, developed urosepsis and the profile of bacterial resistance was similar in the two groups. Hence, treating a positive urine culture in an asymptomatic patient with an indwelling urethral catheter does not reduce the occurrence of urosepsis (25).
Management of Symptomatic Urinary Tract Infections
Choice of Antimicrobial Agents
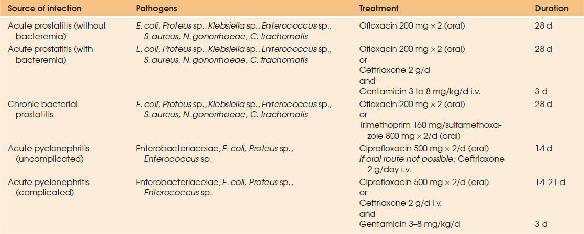
The optimal characteristics of agents to treat UTIs must include activity against the major pathogens involved in these infections, good tissue penetration, and minimal side effects. High urinary levels should be present for an adequate period to eliminate the organisms, since disappearance of bacteriuria is correlated to the sensitivity of the pathogen and to the urine concentration of the antimicrobial agent (74). Inhibitory urinary concentrations are achieved after administration of essentially all commonly used antibiotics. However, an antibiotic achieving active concentrations in the renal tissue is required for infection of the renal tissue; the antibiotic concentration in the serum or plasma can be used as surrogate markers for the antibiotic concentrations in the renal tissue (75). For drugs with concentration-dependent time-kill activity such as the aminoglycosides or the fluoroquinolones, the peak antibiotic concentration is the most important parameter for the in vivo effect. Experimentally, gentamicin and fluoroquinolone treatment are both more effective than b-lactam antibiotics in rapid bacterial killing (Table 88.3.23) (75).
Clinical studies have shown that the renal concentrations of cephalosporins remain higher than the minimal inhibitory concentration for the most common bacteria during the time interval between the administrations of two doses (76–78). In contrast, b-lactam antibiotics with a low pKa and poor lipid solubility penetrate poorly into the prostate, except for some cephalosporins. Good to excellent penetration into the prostatic tissue has been demonstrated with many antimicrobial agents, such as aminoglycosides, fluoroquinolones, sulfonamides, and nitrofurantoin (79).
In ICU patients, the pharmacokinetics of b-lactam and aminoglycosides antibiotics may be profoundly altered due the dynamic and unpredictable pathophysiologic changes that occur in critical illness (80). Consequently, therapeutic drug monitoring may optimize antibiotic therapy (81) especially in septic shock and when continuous renal replacement therapy (CRRT) is used, to individualize dosing and to ensure optimal antibiotic exposure (82). The side effects of treatment should be minimized at both the individual and the community levels. Many patients develop renal failure, associated with inability to concentrate antimicrobial agents in the urine.
Otherwise, antimicrobial treatment should produce a minimal effect on the bacterial flora of the community (83). From this standpoint, there is significant literature demonstrating that the use of fluoroquinolone is associated with the emergence of resistant pathogens (84–87). This implies that an indication for antibacterial therapy should be weighed thoroughly and fluoroquinolones should be used in accordance with sensitivity testing (88). Hence, it is of importance to stress that UTI should not be treated before the results of sensitivity testing, except in patients with pyelonephritis and those with severe sepsis or septic shock who require empirical antimicrobial therapy.
| TABLE 88.3.23 Antimicrobial Treatment of Urinary Tract Infections. Each Empirical Treatment Must Be Adapted to the Susceptibility Testing Results | |||
 | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








