Airway pressures: measured at proximal airways during mechanical ventilation
 Peak inspiratory pressure (PIP)
Peak inspiratory pressure (PIP)
 Maximal pressure measured at end inspiration
Maximal pressure measured at end inspiration
 Determined by elastic and resistive properties of the respiratory system
Determined by elastic and resistive properties of the respiratory system
 Plateau pressure (Pplat)
Plateau pressure (Pplat)
 Estimation of peak alveolar pressure
Estimation of peak alveolar pressure
 Measured by applying end-inspiratory breath hold for 0.5 to 2 seconds (zero flow)
Measured by applying end-inspiratory breath hold for 0.5 to 2 seconds (zero flow)
 Determined by tidal volume and compliance of respiratory system
Determined by tidal volume and compliance of respiratory system
 Measurement only valid during passive inflation (use assist control mode, not pressure support ventilation)
Measurement only valid during passive inflation (use assist control mode, not pressure support ventilation)
 Compliance
Compliance
 Distensibility of system; inverse of elastance
Distensibility of system; inverse of elastance
 Change in volume divided by the pressure required to produce that volume
Change in volume divided by the pressure required to produce that volume
 Respiratory system compliance (compliance of chest wall + lung)
Respiratory system compliance (compliance of chest wall + lung)
 CRS = ΔV/ΔP = VT/(Pplat – PEEP)
CRS = ΔV/ΔP = VT/(Pplat – PEEP)
 Normal value for mechanically ventilated patient is 50 to100 mL/cm H2O
Normal value for mechanically ventilated patient is 50 to100 mL/cm H2O
 Chest wall compliance
Chest wall compliance
 Calculated using esophageal pressure (PES) as surrogate for pleural pressure (PPL)
Calculated using esophageal pressure (PES) as surrogate for pleural pressure (PPL)
 CCW = ΔV/ΔP = VT/ΔPPL
CCW = ΔV/ΔP = VT/ΔPPL
 Normal value = 200 mL/cm H2O
Normal value = 200 mL/cm H2O
 Causes of increased chest wall compliance: paralysis, flail chest
Causes of increased chest wall compliance: paralysis, flail chest
 Causes of decreased chest wall compliance: abdominal distension, thoracic deformities, chest wall burns/edema, increased muscle tone
Causes of decreased chest wall compliance: abdominal distension, thoracic deformities, chest wall burns/edema, increased muscle tone
 Lung compliance
Lung compliance
 Calculated using transpulmonary pressure
Calculated using transpulmonary pressure
 CL = ΔV/ΔP = VT/((Pplat – PEEP) – ΔPPL)
CL = ΔV/ΔP = VT/((Pplat – PEEP) – ΔPPL)
 Normal value = 200 mL/cm H2O
Normal value = 200 mL/cm H2O
 Causes of increased lung compliance: emphysema
Causes of increased lung compliance: emphysema
 Causes of decreased lung compliance: consolidation, atelectasis, pulmonary edema, pulmonary fibrosis, pneumonectomy, mainstem intubation
Causes of decreased lung compliance: consolidation, atelectasis, pulmonary edema, pulmonary fibrosis, pneumonectomy, mainstem intubation
 Resistance
Resistance
 Opposition to air flow within airways
Opposition to air flow within airways
 Change in pressure divided by flow
Change in pressure divided by flow
 Inspiratory resistance:
Inspiratory resistance:
 RI = (PIP – Pplat)/V˙
RI = (PIP – Pplat)/V˙
 Set ventilator for constant inspiratory flow
Set ventilator for constant inspiratory flow
 Normal value for intubated and mechanically ventilated patients < 10 cm H2O/L/s at flow of 1 L/s
Normal value for intubated and mechanically ventilated patients < 10 cm H2O/L/s at flow of 1 L/s
 Causes of increased resistance: bronchospasm, secretions, small endotracheal tube
Causes of increased resistance: bronchospasm, secretions, small endotracheal tube
Pressure-Volume Curves
 Depict static relationship between pressure and volume of respiratory system.
Depict static relationship between pressure and volume of respiratory system.
 Constructed by measuring pressure as lungs are inflated or deflated (different curves due to hysteresis); accurate measurements require sedation and often paralysis.
Constructed by measuring pressure as lungs are inflated or deflated (different curves due to hysteresis); accurate measurements require sedation and often paralysis.
 Normal shape is sigmoidal with lower and upper inflection points.
Normal shape is sigmoidal with lower and upper inflection points.
 Lower inflection point may represent the closing volume of the alveoli. Similarly, the upper inflection point may represent overdistension of the alveoli.
Lower inflection point may represent the closing volume of the alveoli. Similarly, the upper inflection point may represent overdistension of the alveoli.
 Clinical utility of adjusting ventilator to avoid alveolar collapse and overdistension based on pressure-volume curve is not clear.
Clinical utility of adjusting ventilator to avoid alveolar collapse and overdistension based on pressure-volume curve is not clear.
SUGGESTED READINGS
Bigatello LM, Davignon KR, Stelfox HT. Respiratory mechanics and ventilator waveforms in the patient with acute lung injury. Respir Care. 2005;50(2):235-245.
Hess DR, Bigatello LM. The chest wall in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14(1):94-102.
Hess DR, Medoff BD, Fessler MB. Pulmonary mechanics and graphics during positive pressure ventilation. Int Anesthesiol Clin. 1999;37(3):15-34.
Jubran A, Tobin MJ. Monitoring during mechanical ventilation. Clin Chest Med. 1996;17(3):453-473.
Lucangelo U, Bernabè F, Blanch L. Lung mechanics at the bedside: make it simple. Curr Opin Crit Care. 2007;13(1):64-72.
Lucangelo U, Bernabé F, Blanch L. Respiratory mechanics derived from signals in the ventilator circuit. Respir Care. 2005;50(1):55-65.
3.2
Respiratory Failure, Mechanical Ventilation, and Weaning
Jean Kwo and Daniel Chipman
Definitions
 Respiratory failure: inability of the lungs to perform their basic task of gas exchange: the transfer of oxygen from inhaled air into the blood and the transfer of carbon dioxide from the blood into exhaled air
Respiratory failure: inability of the lungs to perform their basic task of gas exchange: the transfer of oxygen from inhaled air into the blood and the transfer of carbon dioxide from the blood into exhaled air
 Type 1 respiratory failure: hypoxemic (PaO2 < 60 mmHg)
Type 1 respiratory failure: hypoxemic (PaO2 < 60 mmHg)
 Due to ventilation/perfusion (V/Q) mismatch or shunt
Due to ventilation/perfusion (V/Q) mismatch or shunt
 Type 2 respiratory failure: hypercarbic (PaCO2 > 45 mmHg)
Type 2 respiratory failure: hypercarbic (PaCO2 > 45 mmHg)
 Due to increased CO2 production (fever, sepsis, burns, overfeeding) or decreased minute ventilation (decreased respiratory rate or tidal volume, increased dead space)
Due to increased CO2 production (fever, sepsis, burns, overfeeding) or decreased minute ventilation (decreased respiratory rate or tidal volume, increased dead space)
Common Causes to Remember
Indications for Mechanical Ventilation
Epidemiology
 Data from study in 2010: 790,257 hospitalizations involving mechanical ventilation in 2005; rate of 2.7 per 1,000 population
Data from study in 2010: 790,257 hospitalizations involving mechanical ventilation in 2005; rate of 2.7 per 1,000 population
 Mortality: 34.5%
Mortality: 34.5%
 Incidence and mortality increases with age.
Incidence and mortality increases with age.
Key Pathophysiology
 Acute respiratory failure can result from pathology affecting any component of the respiratory pathway from the central nervous system (CNS) control centers to the spinal cord to the muscles of respiration to the lungs and their vasculature.
Acute respiratory failure can result from pathology affecting any component of the respiratory pathway from the central nervous system (CNS) control centers to the spinal cord to the muscles of respiration to the lungs and their vasculature.
 Causes are often multifactorial.
Causes are often multifactorial.
 Understanding the cause of the acute respiratory failure is key to treating and reversing it.
Understanding the cause of the acute respiratory failure is key to treating and reversing it.
Differential Diagnosis
 CNS: drugs, sedatives, central sleep apnea, encephalopathy
CNS: drugs, sedatives, central sleep apnea, encephalopathy
 Spinal cord: trauma, transverse myelitis
Spinal cord: trauma, transverse myelitis
 Neuromuscular system: polio, Guillain-Barré syndrome, critical care/steroid myopathy
Neuromuscular system: polio, Guillain-Barré syndrome, critical care/steroid myopathy
 Chest wall/pleural space: kyphosis, obesity, pleural effusion, pneumothorax, hemothorax
Chest wall/pleural space: kyphosis, obesity, pleural effusion, pneumothorax, hemothorax
 Upper airways: obstructive sleep apnea, vocal cord paralysis, tracheomalacia
Upper airways: obstructive sleep apnea, vocal cord paralysis, tracheomalacia
 Lower airways: bronchospasm, chronic obstructive disease, infection, CHF
Lower airways: bronchospasm, chronic obstructive disease, infection, CHF
 Lung parenchyma: infection, interstitial lung disease
Lung parenchyma: infection, interstitial lung disease
 Pulmonary vasculature: pulmonary embolism, pulmonary hypertension
Pulmonary vasculature: pulmonary embolism, pulmonary hypertension
Management and Treatment
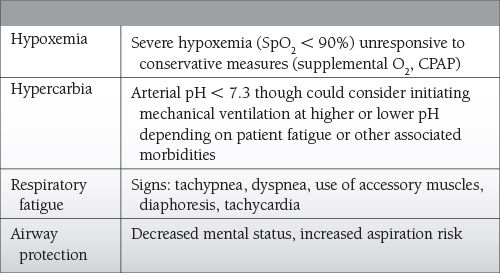
 Mechanical ventilation may be needed to treat hypoxemia, hypercarbia, and respiratory fatigue.
Mechanical ventilation may be needed to treat hypoxemia, hypercarbia, and respiratory fatigue.
 Ventilator settings should be chosen to not only provide adequate oxygenation and ventilation but also to promote patient-ventilator synchrony, maintain alveolar recruitment while avoiding alveolar overdistention, and avoid auto-positive end-expiratory pressure (PEEP) and ventilator-induced lung injury.
Ventilator settings should be chosen to not only provide adequate oxygenation and ventilation but also to promote patient-ventilator synchrony, maintain alveolar recruitment while avoiding alveolar overdistention, and avoid auto-positive end-expiratory pressure (PEEP) and ventilator-induced lung injury.
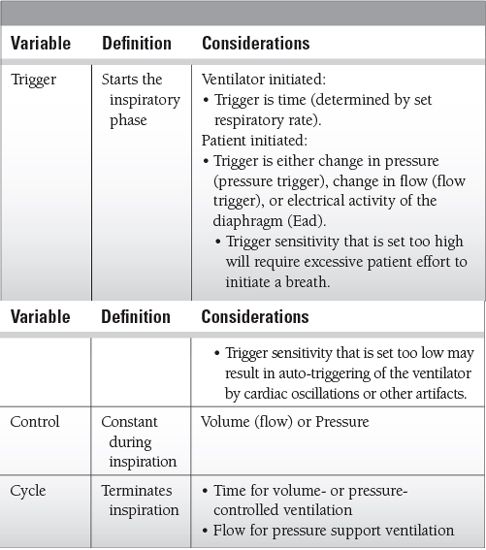
 Understanding the variables that determine how a ventilator breath is delivered, how to set the ventilator, and ventilator modes is essential to avoid the harmful effects of mechanical ventilation.
Understanding the variables that determine how a ventilator breath is delivered, how to set the ventilator, and ventilator modes is essential to avoid the harmful effects of mechanical ventilation.
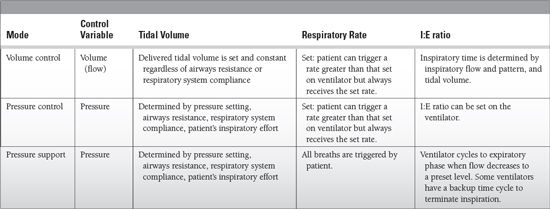
 Ventilator settings
Ventilator settings
 Should be tailored to underlying condition resulting in respiratory failure
Should be tailored to underlying condition resulting in respiratory failure
 Tidal volume
Tidal volume
 Target tidal volume of 4 to 10 mL/kg
Target tidal volume of 4 to 10 mL/kg
 Should use predicted body weight (based on gender and height of patient) to determine tidal volume:
Should use predicted body weight (based on gender and height of patient) to determine tidal volume:
 Males: Predicted Body Weight (PBW) = 50 + 2.3 × (Height (inches) − 60)
Males: Predicted Body Weight (PBW) = 50 + 2.3 × (Height (inches) − 60)
 Females: PBW = 45.5 + 2.3 × (Height (inches) − 60)
Females: PBW = 45.5 + 2.3 × (Height (inches) − 60)
 In patients with acute respiratory distress syndrome (ARDS), acute lung injury (ALI), should decrease tidal volumes to 6 mL/kg or less because lower tidal volumes are associated with a lower mortality rate than higher tidal volumes.
In patients with acute respiratory distress syndrome (ARDS), acute lung injury (ALI), should decrease tidal volumes to 6 mL/kg or less because lower tidal volumes are associated with a lower mortality rate than higher tidal volumes.
 Patients with neuromuscular disease may need higher tidal volumes (8 to 10 mL/kg) to prevent atelectasis.
Patients with neuromuscular disease may need higher tidal volumes (8 to 10 mL/kg) to prevent atelectasis.
 Keep plateau pressures < 30 cm H2O.
Keep plateau pressures < 30 cm H2O.
 Consider decreasing tidal volume if plateau pressure exceeds 28 cm H2O in patients with normal chest wall compliance.
Consider decreasing tidal volume if plateau pressure exceeds 28 cm H2O in patients with normal chest wall compliance.
 Inspiratory time
Inspiratory time
 Set directly in pressure-controlled ventilation
Set directly in pressure-controlled ventilation
 Determined by tidal volume, flow rate, and flow pattern in volume-controlled ventilation
Determined by tidal volume, flow rate, and flow pattern in volume-controlled ventilation
 Determined by cycle off (flow deceleration) sensitivity in pressure support ventilation
Determined by cycle off (flow deceleration) sensitivity in pressure support ventilation
 If set incorrectly (too long or too short) may result in patient/ventilator asynchrony
If set incorrectly (too long or too short) may result in patient/ventilator asynchrony
 Should be set shorter than expiratory time to prevent inverse I:E ratio
Should be set shorter than expiratory time to prevent inverse I:E ratio
 Respiratory rate
Respiratory rate
 Minute ventilation is determined by tidal volume and respiratory rate.
Minute ventilation is determined by tidal volume and respiratory rate.
 Aim for minute ventilation of 8 to 12 L/min (higher with increased CO2 production and/or deadspace).
Aim for minute ventilation of 8 to 12 L/min (higher with increased CO2 production and/or deadspace).
 Adjust rate to achieve desired pH and pCO2.
Adjust rate to achieve desired pH and pCO2.
 High respiratory rates can be associated with air-trapping and auto-PEEP.
High respiratory rates can be associated with air-trapping and auto-PEEP.
 I:E ratio
I:E ratio
 Set directly in pressure-controlled ventilation
Set directly in pressure-controlled ventilation
 Determined by tidal volume, respiratory rate, and flow pattern in volume-controlled ventilation
Determined by tidal volume, respiratory rate, and flow pattern in volume-controlled ventilation
 Longer inspiratory time can result in improved oxygenation but is also associated with auto-PEEP and hemodynamic instability.
Longer inspiratory time can result in improved oxygenation but is also associated with auto-PEEP and hemodynamic instability.
 Expiratory time should be longer than inspiratory time and is determined by inspiratory time and respiratory rate.
Expiratory time should be longer than inspiratory time and is determined by inspiratory time and respiratory rate.
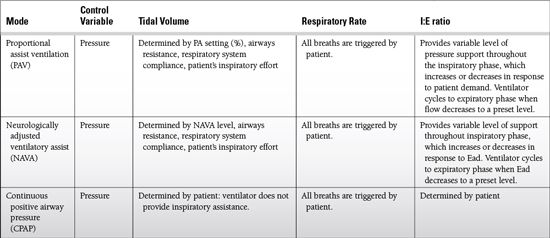
 PEEP
PEEP
 Increases Functional residual capacity (FRC), decreases shunt, improves lung compliance
Increases Functional residual capacity (FRC), decreases shunt, improves lung compliance
 Can titrate by
Can titrate by
 Oxygenation
Oxygenation
 PEEP tables
PEEP tables
 Pressure-volume curves—lower inflexion point
Pressure-volume curves—lower inflexion point
 PEEP trial to determine best respiratory system compliance
PEEP trial to determine best respiratory system compliance
 PEEP titrated using esophageal pressure
PEEP titrated using esophageal pressure
 No evidence for best method of titrating PEEP
No evidence for best method of titrating PEEP
 Excessive PEEP may cause overdistention increasing dead space and reducing lung compliance.
Excessive PEEP may cause overdistention increasing dead space and reducing lung compliance.
 Proportional assist
Proportional assist
 Used with proportional assist ventilation (PAV)
Used with proportional assist ventilation (PAV)
 Determines the percentage of the work of breathing that will be provided by the ventilator
Determines the percentage of the work of breathing that will be provided by the ventilator
 Increasing the level of proportional assist results in decreased work for the patient.
Increasing the level of proportional assist results in decreased work for the patient.
 NAVA level
NAVA level
 Used with neurologically adjusted ventilatory assist (NAVA)
Used with neurologically adjusted ventilatory assist (NAVA)
 Determines the level of support provided by the ventilator
Determines the level of support provided by the ventilator
Common Modes of Mechanical Ventilation
 Indications
Indications
 Pulmonary edema
Pulmonary edema
 Acute COPD exacerbation
Acute COPD exacerbation
 Immunocompromised patient with acute respiratory failure
Immunocompromised patient with acute respiratory failure
 Postextubation in patients at high risk for reintubation
Postextubation in patients at high risk for reintubation
 Chronic heart failure
Chronic heart failure
 Stridor not requiring immediate reintubation
Stridor not requiring immediate reintubation
 Multiple comorbidities
Multiple comorbidities
 Severe COPD
Severe COPD
 Obesity hypoventilation syndrome
Obesity hypoventilation syndrome
 Contraindications
Contraindications
 Cardiac or respiratory arrest
Cardiac or respiratory arrest
 ARDS
ARDS
 Severe encephalopathy (e.g., GCS < 10)
Severe encephalopathy (e.g., GCS < 10)
 Severe upper gastrointestinal bleeding/gastric or esophageal surgery
Severe upper gastrointestinal bleeding/gastric or esophageal surgery
 Hemodynamic instability or unstable cardiac arrhythmia
Hemodynamic instability or unstable cardiac arrhythmia
 Facial surgery, trauma, or deformity
Facial surgery, trauma, or deformity
 Inability to cooperate/protect the airway
Inability to cooperate/protect the airway
 Inability to clear respiratory secretions
Inability to clear respiratory secretions
 High risk for aspiration
High risk for aspiration
 Use of NIV requires frequent reassessment for success or failure. Delay in intubation may result in increased mortality. Consider prompt intubation if there is
Use of NIV requires frequent reassessment for success or failure. Delay in intubation may result in increased mortality. Consider prompt intubation if there is
 Increased accessory muscle use
Increased accessory muscle use
 Increased respiratory rate
Increased respiratory rate
 Worsening arterial blood gas
Worsening arterial blood gas
 Increased dyspnea
Increased dyspnea
 Increased heart rate
Increased heart rate
 Discontinuation of mechanical ventilation
Discontinuation of mechanical ventilation
 Assessment of potential for ventilator discontinuation
Assessment of potential for ventilator discontinuation
 Is there evidence of resolution of underlying disease that led to respiratory failure and need for mechanical ventilation?
Is there evidence of resolution of underlying disease that led to respiratory failure and need for mechanical ventilation?
 Is gas exchange adequate?
Is gas exchange adequate?
 Oxygenation: PaO2/FiO2 > 150 to 200, PEEP < 8 cm H2O, and FiO2 <40% to 50%
Oxygenation: PaO2/FiO2 > 150 to 200, PEEP < 8 cm H2O, and FiO2 <40% to 50%
 Ventilation: pH ≥ 7.25
Ventilation: pH ≥ 7.25
 Is the patient hemodynamically stable (no active myocardial ischemia or clinically important hypotension requiring vasoactive drug therapy)?
Is the patient hemodynamically stable (no active myocardial ischemia or clinically important hypotension requiring vasoactive drug therapy)?
 Can the patient initiate an inspiratory effort?
Can the patient initiate an inspiratory effort?
 Sedation should be adjusted so patient can cooperate with weaning process.
Sedation should be adjusted so patient can cooperate with weaning process.
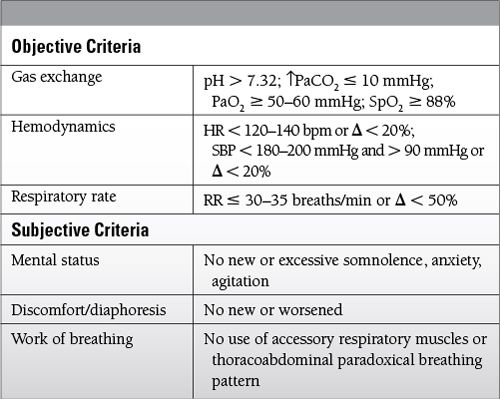
 The spontaneous breathing trial (SBT) is the best predictor of successful ventilation discontinuation.
The spontaneous breathing trial (SBT) is the best predictor of successful ventilation discontinuation.
 SBT can be performed with a T-piece or with ventilator settings PS 0/PEEP 0.
SBT can be performed with a T-piece or with ventilator settings PS 0/PEEP 0.
 A low level of PS (5 to 7 cm H2O) may be used to overcome resistance through an endotracheal tube.
A low level of PS (5 to 7 cm H2O) may be used to overcome resistance through an endotracheal tube.
 Most patients who tolerate an SBT of 30 to 120 minutes can be discontinued successfully from mechanical ventilation.
Most patients who tolerate an SBT of 30 to 120 minutes can be discontinued successfully from mechanical ventilation.
 Criteria to determine tolerance of an SBT
Criteria to determine tolerance of an SBT
 Set ventilator to comfortable, nonfatiguing mode for patient.
Set ventilator to comfortable, nonfatiguing mode for patient.
 Look for cause of failed SBT.
Look for cause of failed SBT.
 Most common cause is need for further resolution of underlying disease.
Most common cause is need for further resolution of underlying disease.
 Other contributory causes include dynamic hyperinflation, myocardial ischemia and cardiac disease, critical illness neuropathy/myopathy.
Other contributory causes include dynamic hyperinflation, myocardial ischemia and cardiac disease, critical illness neuropathy/myopathy.
 Tracheostomy tube malposition should be considered if patient fails to wean off a low level of PS or develops respiratory distress quickly after removal of positive pressure ventilation.
Tracheostomy tube malposition should be considered if patient fails to wean off a low level of PS or develops respiratory distress quickly after removal of positive pressure ventilation.
 For example, bronchoscopic examination might reveal occlusion of airway device by the posterior membrane of the trachea.
For example, bronchoscopic examination might reveal occlusion of airway device by the posterior membrane of the trachea.
 SBT should be repeated once the cause for failed SBT is corrected.
SBT should be repeated once the cause for failed SBT is corrected.
 Use of weaning protocols is associated with reductions in weaning duration, total duration of mechanical ventilation, and ICU length of stay.
Use of weaning protocols is associated with reductions in weaning duration, total duration of mechanical ventilation, and ICU length of stay.
 Criteria for extubation
Criteria for extubation
 Successful completion of SBT
Successful completion of SBT
 Ability to protect airway and clear secretions
Ability to protect airway and clear secretions
 Quality of cough
Quality of cough
 Quantity of secretions
Quantity of secretions
 Frequency of suctioning
Frequency of suctioning
 Absence of upper airway edema
Absence of upper airway edema
 Can occur with prolonged mechanical ventilation, smaller airways (women, children), trauma, repeated or traumatic intubations
Can occur with prolonged mechanical ventilation, smaller airways (women, children), trauma, repeated or traumatic intubations
 May be diagnosed with a leak test and/or direct visualization of airway with laryngoscopy or fiberoptic examination of glottis
May be diagnosed with a leak test and/or direct visualization of airway with laryngoscopy or fiberoptic examination of glottis
 Leak test is performed with patient breathing spontaneously, off positive pressure ventilation, and endotracheal tube cuff deflated.
Leak test is performed with patient breathing spontaneously, off positive pressure ventilation, and endotracheal tube cuff deflated.
 Presence of a leak suggests absence of significant airway swelling.
Presence of a leak suggests absence of significant airway swelling.
 However, absence of a leak is not necessarily an absolute contraindication for extubation.
However, absence of a leak is not necessarily an absolute contraindication for extubation.
 Methylprednisolone 20 mg IV 12 hours before extubation and every 4 hours until tube removal has been shown to decrease the incidence of postextubation laryngeal edema and reintubation secondary to laryngeal edema.
Methylprednisolone 20 mg IV 12 hours before extubation and every 4 hours until tube removal has been shown to decrease the incidence of postextubation laryngeal edema and reintubation secondary to laryngeal edema.
 Patient cooperation
Patient cooperation
 Patient should be awake, alert, comfortable, and able to follow instructions to cough.
Patient should be awake, alert, comfortable, and able to follow instructions to cough.
 Anxiolytics and pain medications may be titrated to achieve above goal.
Anxiolytics and pain medications may be titrated to achieve above goal.
Outcomes
 Mortality associated with mechanical ventilation: 30% to 60% depending on patient population studied.
Mortality associated with mechanical ventilation: 30% to 60% depending on patient population studied.
 Predictors of mortality include advanced age, a medical admitting diagnosis (vs surgical), respiratory failure due to primarily a pulmonary cause, and the inability to wean from mechanical ventilation.
Predictors of mortality include advanced age, a medical admitting diagnosis (vs surgical), respiratory failure due to primarily a pulmonary cause, and the inability to wean from mechanical ventilation.
 Predictors of failure to discontinue mechanical ventilation include a medical admitting diagnosis (vs surgical) and history of underlying respiratory disease.
Predictors of failure to discontinue mechanical ventilation include a medical admitting diagnosis (vs surgical) and history of underlying respiratory disease.
 One study using ICD-9 codes to identify patients receiving mechanical ventilation found that only 30.8% of patients were discharged home from the hospital; 28.2% of patients were discharged to a nursing facility.
One study using ICD-9 codes to identify patients receiving mechanical ventilation found that only 30.8% of patients were discharged home from the hospital; 28.2% of patients were discharged to a nursing facility.
 In another study of Medicare beneficiaries aged ≥ 65, mechanical ventilation during hospitalization was associated with a 1-year mortality of 72% and significant disability.
In another study of Medicare beneficiaries aged ≥ 65, mechanical ventilation during hospitalization was associated with a 1-year mortality of 72% and significant disability.
 Estimated costs were $27 billion nationally or 12% of all hospital costs.
Estimated costs were $27 billion nationally or 12% of all hospital costs.
SUGGESTED READINGS
Barnato A, Albert S, Angus D, et al. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183: 1037-1042.
Bigatello L, Stelfox H, Berra L, et al. Outcome of patients undergoing prolonged mechanical ventilation after critical illness. Crit Care Med. 2007;35:2491-2249.
Blackwood B, Alderdice F, Burns K, et al. Use of weaning protocols for reducing duration of mechanical ventilation in critically ill adult patients: cochrane systematic review and meta-analysis. BMJ. 2011;342:c7237.
de la Olivia P, Schuffelmann C, Gomez-Zamora A, et al. Asynchrony, neural drive, ventilatory variability and COMFORT: NAVA versus pressure support in pediatrics. A non-randomized cross-over trial. Intensive Care Med. 2012;38:838-846.
Esan A, Hess D, Raoof S, et al. Severe hypoxemic respiratory failure: ventilation strategies. Chest. 2010;137:1203-1216.
Estaban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332:345-350.
Esteban A, Frutos F, Ferguson ND, et al. Noninvasive positive pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452-2460.
Francois B, Bellissant E, Gissot V, et al. 12-h pretreatment with methylprednisolone versus placebo for prevention of postextubation laryngeal oedema: a randomized double-blind trial. Lancet. 2007;369:1083-1089.
Grasso S, Puntillo F, Mascia L, et al. Compensation for increase in respiratory workload during mechanical ventilation. Pressure-support versus proportional-assist ventilation. Am J Respir Crit Care Med. 2000; 161(3 Pt 1):819-826.
MacIntyre N. Current issues in mechanical ventilation for respiratory failure. Chest. 2005;128:561S–567S.
MacIntyre NR, Cook DJ, Ely EW, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support. Chest. 2001;120: 375S-395S.
Mercat A, Richard J, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;200: 637-645.
NIH/NHLBI ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute long injury and acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
Sinderby C, Navalesi P, Beck J, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999;5:1433-1436.
Wunsch H, Linde-Zwirble W, Angus D, et al. Epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38: 1947-1953.
Acute Respiratory Distress Syndrome and Acute Lung Injury
Sheri Berg
Introduction
Syndrome of respiratory failure is characterized by
 Onset of acute respiratory is distress syndrome (ARDS)
Onset of acute respiratory is distress syndrome (ARDS)
 PaO2/FiO2 ≤200 (ARDS) and ≤300 Acute Lung Injury (ALI)
PaO2/FiO2 ≤200 (ARDS) and ≤300 Acute Lung Injury (ALI)
 Bilateral (patchy, diffuse, or homogenous) infiltrates in chest x-ray (CXR)
Bilateral (patchy, diffuse, or homogenous) infiltrates in chest x-ray (CXR)
 No clinical evidence of left atrial hypertension (diagnosis no longer requires pulmonary capillary wedge pressure [PCWP]; ECHO can be used to diagnose systolic or diastolic dysfunction as a cause of pulmonary edema).
No clinical evidence of left atrial hypertension (diagnosis no longer requires pulmonary capillary wedge pressure [PCWP]; ECHO can be used to diagnose systolic or diastolic dysfunction as a cause of pulmonary edema).
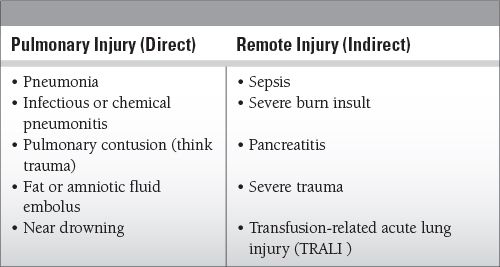
Common Causes to Remember
Epidemiology
 Affects more than 200,000 people in the United States per year
Affects more than 200,000 people in the United States per year
 Associated mortality is 30% to 35%.
Associated mortality is 30% to 35%.
Key Pathophysiology
Systemic inflammatory response that injures the lungs (and likely other organs as well); leads to the classic “diffuse alveolar damage”:
 Alveolar injury: involves all aspects of the alveolar-capillary interface; damage to the type 2 cells amplifies the inflammatory response (remember, these are the surfactant-producing pneumocytes that are also responsible for clearing edema via fluid transport).
Alveolar injury: involves all aspects of the alveolar-capillary interface; damage to the type 2 cells amplifies the inflammatory response (remember, these are the surfactant-producing pneumocytes that are also responsible for clearing edema via fluid transport).
 Exudative phase: interstitial and alveolar edema develop from the increased permeability of damaged endothelium; exudates eventually line the alveolar walls with the characteristic hyaline membranes; the combination of edema, collapse, and consolidation leads to decreased lung compliance and produces hypoxemia (hallmarks of ARDS).
Exudative phase: interstitial and alveolar edema develop from the increased permeability of damaged endothelium; exudates eventually line the alveolar walls with the characteristic hyaline membranes; the combination of edema, collapse, and consolidation leads to decreased lung compliance and produces hypoxemia (hallmarks of ARDS).
 Fibroproliferative phase: acquisition of chronic inflammatory characteristics can occur within 1 to 2 weeks; fibroblasts and collagen deposits lead to destruction of air spaces and interstitial fibrosis.
Fibroproliferative phase: acquisition of chronic inflammatory characteristics can occur within 1 to 2 weeks; fibroblasts and collagen deposits lead to destruction of air spaces and interstitial fibrosis.
 Pulmonary edema contributes to hypoxemia via shunting (perfusion, no ventilation) and decreases compliance as the lungs “stiffen.”
Pulmonary edema contributes to hypoxemia via shunting (perfusion, no ventilation) and decreases compliance as the lungs “stiffen.”
 Can attempt to increase PaO2 via recruitment of nonventilated alveoli (PEEP) and increasing the FiO2
Can attempt to increase PaO2 via recruitment of nonventilated alveoli (PEEP) and increasing the FiO2
 Hypoxic pulmonary vasoconstriction (PA constriction in the setting of hypoxia to divert blood away from hypoventilated alveoli) is a naturally occurring phenomenon that helps to attenuate hypoxemia.
Hypoxic pulmonary vasoconstriction (PA constriction in the setting of hypoxia to divert blood away from hypoventilated alveoli) is a naturally occurring phenomenon that helps to attenuate hypoxemia.
 Decreased pulmonary blood flow, vascular occlusion, and airway overdistention can create areas of dead space and lead to hypercapnia.
Decreased pulmonary blood flow, vascular occlusion, and airway overdistention can create areas of dead space and lead to hypercapnia.
 Thrombosis of small arteries and remodeling often obliterates sections of pulmonary vasculature, which can lead to worsening edema, pulmonary hypertension, and progression to right heart failure.
Thrombosis of small arteries and remodeling often obliterates sections of pulmonary vasculature, which can lead to worsening edema, pulmonary hypertension, and progression to right heart failure.
Differential Diagnosis
A number of noninfectious processes mimic ARDS:
 Acute eosinophilic pneumonia: can also see severe hypoxemia (PaO2 < 60 mmHg), diffuse pulmonary infiltrates—as in ARDS—but can be diagnosed by an increased number of eosinophils in bronchoalveolar lavage (BAL) fluid; treatment with corticosteroids results in rapid reversal of respiratory failure.
Acute eosinophilic pneumonia: can also see severe hypoxemia (PaO2 < 60 mmHg), diffuse pulmonary infiltrates—as in ARDS—but can be diagnosed by an increased number of eosinophils in bronchoalveolar lavage (BAL) fluid; treatment with corticosteroids results in rapid reversal of respiratory failure.
 Acute cryptogenic organizing pneumonia (COP)/bronchiolitis obliterans organizing pneumonia (BOOP): hypoxemia, patchy pulmonary infiltrates, BAL will reveal a predominance of lymphocytes; alveolar exudates can lead to fibrosis; patients may have underlying chronic inflammatory disease (which can help with the diagnosis of COP/BOOP); treat with corticosteroids.
Acute cryptogenic organizing pneumonia (COP)/bronchiolitis obliterans organizing pneumonia (BOOP): hypoxemia, patchy pulmonary infiltrates, BAL will reveal a predominance of lymphocytes; alveolar exudates can lead to fibrosis; patients may have underlying chronic inflammatory disease (which can help with the diagnosis of COP/BOOP); treat with corticosteroids.
 Acute hypersensitivity pneumonitis: results from inhalation of particulate matter, hypoxemia, bilateral opacities can be observed; differentiated from ARDS as there is a clear inciting agent; treat with corticosteroids.
Acute hypersensitivity pneumonitis: results from inhalation of particulate matter, hypoxemia, bilateral opacities can be observed; differentiated from ARDS as there is a clear inciting agent; treat with corticosteroids.
 Diffuse alveolar hemorrhage: flooding of alveoli with blood and BAL will be grossly sanguineous; hypoxemia (can be severe); usually an underlying vasculitis; mainstay of treatment is corticosteroids and immunosuppressive agents.
Diffuse alveolar hemorrhage: flooding of alveoli with blood and BAL will be grossly sanguineous; hypoxemia (can be severe); usually an underlying vasculitis; mainstay of treatment is corticosteroids and immunosuppressive agents.
Management and Treatment
 Diagnose and begin to treat the underlying condition; support hemodynamics, treat infections; support other organ systems while the lungs recover.
Diagnose and begin to treat the underlying condition; support hemodynamics, treat infections; support other organ systems while the lungs recover.
 THE VENT: initiate lung-protective ventilation to help minimize further injury to undamaged alveoli.
THE VENT: initiate lung-protective ventilation to help minimize further injury to undamaged alveoli.
 Small tidal volumes (≤6 cc/kg), low airway pressures (plateau pressures < 30 cm H2O) may improve survival = “ARDS – net protective ventilation.”
Small tidal volumes (≤6 cc/kg), low airway pressures (plateau pressures < 30 cm H2O) may improve survival = “ARDS – net protective ventilation.”
 As this mechanical ventilation strategy (low tidal volumes and plateau pressures) often results in a concomitant respiratory acidosis, permissive hypercapnia may need to be tolerated. If the respiratory rate cannot be increased further (shoot for pH ~7.3 and PaCO2 < 55; however, this is not always possible, especially if there is an underlying metabolic acidosis (as seen in sepsis)) and a severe acidosis develops, then the patient may require renal replacement therapy (CVVH) to help correct the acidosis; should be seriously considered once the pH < 7.2.
As this mechanical ventilation strategy (low tidal volumes and plateau pressures) often results in a concomitant respiratory acidosis, permissive hypercapnia may need to be tolerated. If the respiratory rate cannot be increased further (shoot for pH ~7.3 and PaCO2 < 55; however, this is not always possible, especially if there is an underlying metabolic acidosis (as seen in sepsis)) and a severe acidosis develops, then the patient may require renal replacement therapy (CVVH) to help correct the acidosis; should be seriously considered once the pH < 7.2.
 Improved lung function, decreased duration of mechanical ventilation, and lower number of deaths from hypoxia with moderate (vs low) PEEP levels; “Best PEEP” measurements can be made, although they do not appear to improve outcome.
Improved lung function, decreased duration of mechanical ventilation, and lower number of deaths from hypoxia with moderate (vs low) PEEP levels; “Best PEEP” measurements can be made, although they do not appear to improve outcome.
 Although there is not enough evidence to support routine usage, PEEP can also be titrated to positive transpulmonary pressure via estimation from an esophageal balloon catheter.
Although there is not enough evidence to support routine usage, PEEP can also be titrated to positive transpulmonary pressure via estimation from an esophageal balloon catheter.
 Routine use of recruitment maneuvers has not been shown to be beneficial (and can further compromise patients who are gravely hemodynamically unstable), although it may be worth a try in patients with refractory hypoxemia.
Routine use of recruitment maneuvers has not been shown to be beneficial (and can further compromise patients who are gravely hemodynamically unstable), although it may be worth a try in patients with refractory hypoxemia.
 The underlying goal is prevention of ventilator-induced lung injury (VILI), which can potentially provide added damage to the alveoli.
The underlying goal is prevention of ventilator-induced lung injury (VILI), which can potentially provide added damage to the alveoli.
 Types of VILI include:
Types of VILI include:
 Barotrauma: excessive airway pressures
Barotrauma: excessive airway pressures
 Volutrauma: overdistention of alveoli
Volutrauma: overdistention of alveoli
 Atelectrauma: opening alveoli (inspiration) and collapse (expiration) cause shear injury
Atelectrauma: opening alveoli (inspiration) and collapse (expiration) cause shear injury
 Biotrauma: release of proinflammatory cytokines from disproportionate mechanical forces on the lung
Biotrauma: release of proinflammatory cytokines from disproportionate mechanical forces on the lung
 Conservative fluid management/restriction has been shown to be beneficial in ALI/ARDS, with associated improvement of lung function, respiratory mechanics, gas exchange, and minimizing pulmonary edema.
Conservative fluid management/restriction has been shown to be beneficial in ALI/ARDS, with associated improvement of lung function, respiratory mechanics, gas exchange, and minimizing pulmonary edema.
 “Proning”: it is thought that the prone position initiates redistribution of ventilation to previously collapsed areas of the lung; it has been shown that many patients demonstrate improved oxygenation; however, the effects are transient and mortality is not reduced.
“Proning”: it is thought that the prone position initiates redistribution of ventilation to previously collapsed areas of the lung; it has been shown that many patients demonstrate improved oxygenation; however, the effects are transient and mortality is not reduced.
 Remember: hemodynamic instability can rapidly ensue when patients are prone and extreme vigilance must be employed with regard to maintenance of the secured airway.
Remember: hemodynamic instability can rapidly ensue when patients are prone and extreme vigilance must be employed with regard to maintenance of the secured airway.
 To date, its use is inconclusive and has not been shown to decrease time spent on the ventilator or increase survival.
To date, its use is inconclusive and has not been shown to decrease time spent on the ventilator or increase survival.
 Neuromuscular blockade (NMB)
Neuromuscular blockade (NMB)
 A recent study concluded that initiation of neuromuscular blockade in patients meeting ARDS criteria may be of benefit, as mortality was reduced with the use of cisatracurium for 48 hours; the benefit is likely due to improvement of gas exchange via enhancement of patient-ventilator synchrony and thus, the ability to provide proficient lung-protective ventilation.
A recent study concluded that initiation of neuromuscular blockade in patients meeting ARDS criteria may be of benefit, as mortality was reduced with the use of cisatracurium for 48 hours; the benefit is likely due to improvement of gas exchange via enhancement of patient-ventilator synchrony and thus, the ability to provide proficient lung-protective ventilation.
 There is concern of prolonged weakness and myopathy of critical illness; however, the study failed to demonstrate an increase in myopathy (this is a well-known consequence with an increased incidence associated with the use of steroid neuromuscular blockers).
There is concern of prolonged weakness and myopathy of critical illness; however, the study failed to demonstrate an increase in myopathy (this is a well-known consequence with an increased incidence associated with the use of steroid neuromuscular blockers).
 Other drugs
Other drugs
 Nitric oxide will improve oxygenation and decrease pulmonary vascular resistance, but the effects are temporary and are usually a bridge to extracorporeal membrane oxygenation (ECMO) which can allow the lungs to “rest”; unfortunately, neither has rendered a survival benefit in patients with ARDS.
Nitric oxide will improve oxygenation and decrease pulmonary vascular resistance, but the effects are temporary and are usually a bridge to extracorporeal membrane oxygenation (ECMO) which can allow the lungs to “rest”; unfortunately, neither has rendered a survival benefit in patients with ARDS.
 Steroids were postulated to help attenuate inflammation (which is thought to be involved in the pathogenesis of ARDS); however, they failed to demonstrate any benefit on survival (one study showed increased mortality when steroids were started 14 days after the onset of ALI/ARDS).
Steroids were postulated to help attenuate inflammation (which is thought to be involved in the pathogenesis of ARDS); however, they failed to demonstrate any benefit on survival (one study showed increased mortality when steroids were started 14 days after the onset of ALI/ARDS).
 Other drugs that have been studied and have failed to improve survival include NSAIDs, antioxidants, ketoconazole, surfactant, neutrophil elastase inhibitors, and pentoxyfilline.
Other drugs that have been studied and have failed to improve survival include NSAIDs, antioxidants, ketoconazole, surfactant, neutrophil elastase inhibitors, and pentoxyfilline.
 Current research is evaluating the possible benefits of statins and antiplatelet drugs in ARDS.
Current research is evaluating the possible benefits of statins and antiplatelet drugs in ARDS.
 Salvage therapies
Salvage therapies
 High frequency ventilation: more often used in the neonate and pediatric population; very small tidal volumes with fixed mean airway pressures and high respiratory rates; airway pressures remain low in hopes of minimizing VILI; however, short expiratory times can lead to auto-PEEP (which will not be reflected when measuring airway pressures).
High frequency ventilation: more often used in the neonate and pediatric population; very small tidal volumes with fixed mean airway pressures and high respiratory rates; airway pressures remain low in hopes of minimizing VILI; however, short expiratory times can lead to auto-PEEP (which will not be reflected when measuring airway pressures).
 Inverse ratio ventilation: extends the inspiratory time concept with aims at improving gas exchange at lower levels of PEEP; can also lead to auto-PEEP, adverse hemodynamic effects, and need for sedation in most patients.
Inverse ratio ventilation: extends the inspiratory time concept with aims at improving gas exchange at lower levels of PEEP; can also lead to auto-PEEP, adverse hemodynamic effects, and need for sedation in most patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 If patient fails
If patient fails 