One of the oldest surgical procedures, a tracheostomy is performed in order to gain access to the airway when endotracheal intubation is either contraindicated or impossible.
Indications
 Tracheostomy can be performed electively or emergently (Box 27.1.1).
Tracheostomy can be performed electively or emergently (Box 27.1.1).
 Any lesion or abnormality causing upper-airway obstruction may require a tracheostomy.
Any lesion or abnormality causing upper-airway obstruction may require a tracheostomy.
 If prolonged mechanical ventilation is expected, changing from an endotracheal tube to a tracheostomy may have benefits (described in section on Timing).
If prolonged mechanical ventilation is expected, changing from an endotracheal tube to a tracheostomy may have benefits (described in section on Timing).
Complications
 The most severe complication of tracheostomy is failure to establish an airway, resulting in hypoxemia, brain damage, or death.
The most severe complication of tracheostomy is failure to establish an airway, resulting in hypoxemia, brain damage, or death.
Box 27.1.1 Indications for tracheostomy
Elective
• Upper airway obstruction (impending)
• Neurologic
• Pulmonary
Emergency
• Upper airway obstruction (acute)
• Tumor
• Trauma
• Failed intubation
 Percutaneous tracheostomy tubes can become obstructed by the posterior membranous trachea, or rarely, placement can cause perforation of the posterior wall of the trachea.
Percutaneous tracheostomy tubes can become obstructed by the posterior membranous trachea, or rarely, placement can cause perforation of the posterior wall of the trachea.
 Massive hemorrhage due to a tracheoartery (innominate artery) fistula is a rare but devastating complication:
Massive hemorrhage due to a tracheoartery (innominate artery) fistula is a rare but devastating complication:
 Often there is a sentinel bleed of 100 to 300 mL, which then stops.
Often there is a sentinel bleed of 100 to 300 mL, which then stops.
 This should be recognized and a cardiothoracic surgeon should be consulted immediately. CT angiography or bronchoscopy may be needed to confirm the diagnosis.
This should be recognized and a cardiothoracic surgeon should be consulted immediately. CT angiography or bronchoscopy may be needed to confirm the diagnosis.
 The incidence is less than 1% in several studies, likely due to the low-pressure cuffs used today.
The incidence is less than 1% in several studies, likely due to the low-pressure cuffs used today.
 See Box 27.1.2 for a complete list of complications with tracheostomy.
See Box 27.1.2 for a complete list of complications with tracheostomy.
Equipment
 Tracheostomy tubes can be
Tracheostomy tubes can be
 Plastic (polyvinyl chloride or silicone) or metal (silver or stainless steel)
Plastic (polyvinyl chloride or silicone) or metal (silver or stainless steel)
 Uncuffed or cuffed
Uncuffed or cuffed
 Unfenestrated or fenestrated
Unfenestrated or fenestrated
 A cuff provides an airway seal and reduces aspiration of secretions.
A cuff provides an airway seal and reduces aspiration of secretions.
Box 27.1.2 Complications of tracheostomy
• Failure to establish an airway
• Accidental extubation
• Subcutaneous emphysema
• Pneumothorax
• Pneumomediastinum
• Aspiration
• Bleeding
• Infection
• Tracheal granulations
• Subglottic stenosis
• Tracheal stenosis
• Tracheoarterial fistula
• Posterior tracheal wall perforation
 Modern cuffs are typically low pressure and high volume, and cuff pressures should not exceed 25 cm H2O.
Modern cuffs are typically low pressure and high volume, and cuff pressures should not exceed 25 cm H2O.
 Fenestrated tubes have an additional opening in the posterior portion above the cuff, which should permit upper airway airflow and facilitate speech.
Fenestrated tubes have an additional opening in the posterior portion above the cuff, which should permit upper airway airflow and facilitate speech.
 Other specifications include the outer diameter, inner diameter, and length (angle or curved, standard or extra length, fixed length, or adjustable flange).
Other specifications include the outer diameter, inner diameter, and length (angle or curved, standard or extra length, fixed length, or adjustable flange).
 Adjustment in horizontal or vertical length can be helpful in patients with large necks or tracheal anomalies, respectively.
Adjustment in horizontal or vertical length can be helpful in patients with large necks or tracheal anomalies, respectively.
 Tubes can have a single or dual cannula:
Tubes can have a single or dual cannula:
 Dual-cannula tubes possess an inner cannula and will not connect to a ventilator without it.
Dual-cannula tubes possess an inner cannula and will not connect to a ventilator without it.
 The supposed advantage is quicker and easier cleaning of the inner tube to prevent obstruction by secretions.
The supposed advantage is quicker and easier cleaning of the inner tube to prevent obstruction by secretions.
 Evidence that this decreases pneumonia is lacking.
Evidence that this decreases pneumonia is lacking.
 Disadvantages include a smaller internal diameter, which may increase the work of breathing and paradoxically trap secretions.
Disadvantages include a smaller internal diameter, which may increase the work of breathing and paradoxically trap secretions.
 Tracheostomy tubes can typically be changed after 5 to 7 days of insertion, when there is a mature tract from the skin to the airway.
Tracheostomy tubes can typically be changed after 5 to 7 days of insertion, when there is a mature tract from the skin to the airway.
 If a tracheostomy tube becomes dislodged before there is a mature tract, no immediate attempt should be made to replace the tube, and the patient should first be orotracheally intubated to control the airway.
If a tracheostomy tube becomes dislodged before there is a mature tract, no immediate attempt should be made to replace the tube, and the patient should first be orotracheally intubated to control the airway.
Procedure
 Bedside percutaneous tracheostomy has become an alternative to standard operative tracheostomy performed at the bedside or in the operating room
Bedside percutaneous tracheostomy has become an alternative to standard operative tracheostomy performed at the bedside or in the operating room
 Successful performance of the bedside percutaneous procedure is related to expertise of the operator and ancillary personnel. It appears to be safe when performed by surgeons or well-trained intensivists.
Successful performance of the bedside percutaneous procedure is related to expertise of the operator and ancillary personnel. It appears to be safe when performed by surgeons or well-trained intensivists.
 In comparison to standard operative tracheostomy, bedside percutaneous tracheostomy offers several advantages: it requires less time to perform, it is less expensive, and it is typically performed sooner (because an operating room doesn’t have to be scheduled).
In comparison to standard operative tracheostomy, bedside percutaneous tracheostomy offers several advantages: it requires less time to perform, it is less expensive, and it is typically performed sooner (because an operating room doesn’t have to be scheduled).
 Complication rates are similar with both the procedures.
Complication rates are similar with both the procedures.
 Relative contraindications to percutaneous tracheostomy include age under 15 years, uncorrectable bleeding diathesis, gross distortion of neck, clinically suspected tracheomalacia, infection in soft tissues of neck, obesity and/or short neck which obscures landmarks, or inability to extend neck (cervical spine instability or fusion).
Relative contraindications to percutaneous tracheostomy include age under 15 years, uncorrectable bleeding diathesis, gross distortion of neck, clinically suspected tracheomalacia, infection in soft tissues of neck, obesity and/or short neck which obscures landmarks, or inability to extend neck (cervical spine instability or fusion).
 Percutaneous tracheostomy has been performed successfully by skilled operators in patients who were very old, morbidly obese, had a history of previous tracheostomy, or had thrombocytopenia.
Percutaneous tracheostomy has been performed successfully by skilled operators in patients who were very old, morbidly obese, had a history of previous tracheostomy, or had thrombocytopenia.
Outcomes after Tracheostomy
 There are several putative benefits of subglottic ventilation (Box 27.1.3).
There are several putative benefits of subglottic ventilation (Box 27.1.3).
 Overall, the current evidence supports consistent morbidity benefits of tracheostomy, however no mortality benefits have been proven.
Overall, the current evidence supports consistent morbidity benefits of tracheostomy, however no mortality benefits have been proven.
Box 27.1.3 Putative benefits of tracheostomy
• Decrease in ventilator dead space
• Decreased airway resistance
• Ease of suctioning
• Reduced orolabial and laryngeal trauma
• Increased patient comfort
• Reduced requirement for sedation
• Increased patient mobility
• Shorter duration of mechanical ventilation
• Ability to transfer spontaneously breathing patients to non-ICU settings
• Ease of tube replacement (once tract matures)
• Increased ability for the patient to communicate
• Variable capacity for oral intake of nutrition and medication
Other Considerations
 Timing of Tracheostomy in Endotracheally Intubated Patients
Timing of Tracheostomy in Endotracheally Intubated Patients
 Many physicians believe patients should not be ventilated via an endotracheal tube for longer than 3 weeks unless they are unstable or unlikely to benefit from tracheostomy.
Many physicians believe patients should not be ventilated via an endotracheal tube for longer than 3 weeks unless they are unstable or unlikely to benefit from tracheostomy.
 This view is based upon observations that tracheostomy enhances nursing care, improves patient comfort, and improves patient communication.
This view is based upon observations that tracheostomy enhances nursing care, improves patient comfort, and improves patient communication.
 There is conflicting data about whether early tracheostomy improves outcomes.
There is conflicting data about whether early tracheostomy improves outcomes.
 The best evidence suggests early tracheostomy may improve short-term clinical outcomes such as ventilator days and ICU days, but at a cost of surgical and stoma-related complications.
The best evidence suggests early tracheostomy may improve short-term clinical outcomes such as ventilator days and ICU days, but at a cost of surgical and stoma-related complications.
 A recent multicenter randomized controlled trial looked at early (7 days) versus late (14 days) tracheostomy and found a decrease in ventilator days, a decrease in ICU days, and a trend toward less pneumonia in the early group. No difference in survival was seen, nor did early tracheostomy change any outcomes at 1 year.
A recent multicenter randomized controlled trial looked at early (7 days) versus late (14 days) tracheostomy and found a decrease in ventilator days, a decrease in ICU days, and a trend toward less pneumonia in the early group. No difference in survival was seen, nor did early tracheostomy change any outcomes at 1 year.
 Based on current data, the timing of tracheostomy should be individualized to each patient and must reflect his or her anticipated clinical status, readiness to wean from the ventilator, and possible benefits from tracheostomy.
Based on current data, the timing of tracheostomy should be individualized to each patient and must reflect his or her anticipated clinical status, readiness to wean from the ventilator, and possible benefits from tracheostomy.
 Emergency tracheostomy can usually not be achieved as quickly as cricothyroidotomy, but may be appropriate in patients with an urgent need for a definitive airway that cannot be orotracheally or nasotracheally intubated, but are still oxygenating and ventilating.
Emergency tracheostomy can usually not be achieved as quickly as cricothyroidotomy, but may be appropriate in patients with an urgent need for a definitive airway that cannot be orotracheally or nasotracheally intubated, but are still oxygenating and ventilating.
 Weaning and Decannulation
Weaning and Decannulation
 When there is no longer a need for airway protection and mechanical ventilation, patients may have their tracheostomy tubes downsized.
When there is no longer a need for airway protection and mechanical ventilation, patients may have their tracheostomy tubes downsized.
 In addition, a fenestrated or cuffless tube will allow increased airflow through or around the tube, permitting the tracheostomy tube to be “capped-off” and facilitating speech.
In addition, a fenestrated or cuffless tube will allow increased airflow through or around the tube, permitting the tracheostomy tube to be “capped-off” and facilitating speech.
 A one-way valve, such as a Passy-Muir valve, will also permit laryngeal airflow during expiration and enable speech.
A one-way valve, such as a Passy-Muir valve, will also permit laryngeal airflow during expiration and enable speech.
 Specific weaning strategies are institution dependent: some consider it after the tracheostomy tube has been capped for 48 hours, whereas others consider it once a speaking-valve is tolerated.
Specific weaning strategies are institution dependent: some consider it after the tracheostomy tube has been capped for 48 hours, whereas others consider it once a speaking-valve is tolerated.
 It is important to never place a speaking-valve or cap a nonfenestrated tube with the cuff inflated, as this causes complete airway obstruction.
It is important to never place a speaking-valve or cap a nonfenestrated tube with the cuff inflated, as this causes complete airway obstruction.
 It is standard practice when decannulating a patient to simply remove the tracheostomy tube and allow the wound to heal by secondary intention.
It is standard practice when decannulating a patient to simply remove the tracheostomy tube and allow the wound to heal by secondary intention.
 If the skin is closed primarily and the trachea remains open, any air leak that ensues can cause complications with subcutaneous emphysema, pneumomediastinum, and pneumothorax.
If the skin is closed primarily and the trachea remains open, any air leak that ensues can cause complications with subcutaneous emphysema, pneumomediastinum, and pneumothorax.
SUGGESTED READINGS
Al-Ansara MA, Hijazi MH. Clinical review: Percutaneous dilational tracheostomy. Crit Care. 2006;10:202.
Benumof JL. Airway Management Elsevier health sciences: Principles and Practice. 1st ed. 1996.
Bishop MJ. The timing of tracheotomy: An evolving consensus. Chest. 1989;96(4):712.
Diaz-Reganon G, Minambres E, Ruiz A, et al. Safety and complications of percutaneous tracheostomy in a cohort of 800 mixed ICU patients. Anaesthesia. 2008;63:1198.
Dunn PF, Goulet RL. Endotracheal tubes and airway appliances. Int Anesthesiol Clin. 2000;38:65.
Engels PT, Bagshaw SM, Meier M, Brindley PG. Tracheostomy: From insertion to decannulation. Can J Surg. 2009;52:427.
Fikkers BG, van Veen JA, Kooloos JG, et al. Emphysema and pneumothorax after percutaneous tracheostomy: case reports and an anatomic study. Chest. 2004;125:1805.
Freeman BD, Isabella K, Lin N et al. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000;118:1412.
Futran ND, Dutcher PO, Robert JK. The safety and efficacy of bedside tracheotomy. Otolaryngol Head Neck Surg. 1993;109:707.
Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330(7502):1243.
Hess DR. Tracheostomy tubes and related appliances. Resp Care. 2005;50:497.
Heyrosa MG, Melniczek DM, et al. Percutaneous tracheostomy: a safe procedure in the morbidly obese. J Am Coll Surg. 2006;202:618.
Jones JW, Reynolds M, Hewitt RL, Drapanas T. Tracheo-innominate artery erosion: Successful surgical management of a devastating complication. Ann Surg. 1976;184:194.
Kluge S, Meyer A, Kühnelt P et al. Percutaneous tracheostomy is safe in patients with severe thrombocytopenia. Chest. 2004;126:547.
Loh KS, Irish JC. Traumatic complications of intubation and other airway management procedures. Anesthesiol Clin North Am. 2002;20:953.
Meyer M, Critchlow J, Mansharamani N et al. Repeat bedside percutaneous dilational tracheostomy is a safe procedure. Crit Care Med. 2002;30:986.
Seder DB, Lee K, Rahman C, et al. Safety and feasibility of percutaneous tracheostomy performed by neurointensivists. Neurocrit Care. 2009;10:264.
St John RE, Malen JF. Contemporary issues in adult tracheostomy management. Crit Care Nurs Clin North Am. 2004;16:413.
Stone DJ, Bogdonoff DL. Airway considerations in the management of patients requiring long-term endotracheal intubation. Anesth Analg. 1992;74(2):276.
Terragni PP, Antonelli M, Fumagalli R et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: A randomized controlled trial. JAMA. 2010;303(15):1483.
Trottier SJ, Ritter S, Lakshmanan R, et al. Percutaneous tracheostomy tube obstruction: warning. Chest. 2002;122:1377.
Warren WH. Percutaneous dilational tracheostomy: A note of caution. Crit Care Med. 2000;28:1664.
27.2
Cricothyroidotomy
William Schoenfeld
Cricothyroidotomy (Cricothyrotomy)
 A procedure to surgically introduce an endotracheal tube into the airway through an incision made in the cricothyroid membrane
A procedure to surgically introduce an endotracheal tube into the airway through an incision made in the cricothyroid membrane
Epidemiology
 5 to 35 of 10,000 patients (0.05% to 0.35%) cannot be endotracheally intubated.
5 to 35 of 10,000 patients (0.05% to 0.35%) cannot be endotracheally intubated.
 0.01 to 2 of 10,000 patients are difficult to mask ventilate and intubate.
0.01 to 2 of 10,000 patients are difficult to mask ventilate and intubate.
 Cricothyroidotomy comprises 1% of all intubations in the emergency department and up to 10.9% of intubations in the prehospital setting.
Cricothyroidotomy comprises 1% of all intubations in the emergency department and up to 10.9% of intubations in the prehospital setting.
Indications and Contraindications
 Cricothyroidotomy is indicated when orotracheal or nasotracheal intubation is either unsuccessful or contraindicated, but definitive airway control is urgently required. Tracheostomy (see Section 27.1) is usually preferred when time and the clinical scenario allow.
Cricothyroidotomy is indicated when orotracheal or nasotracheal intubation is either unsuccessful or contraindicated, but definitive airway control is urgently required. Tracheostomy (see Section 27.1) is usually preferred when time and the clinical scenario allow.
 Patients with traumatic facial fractures
Patients with traumatic facial fractures
 Profound hemorrhage or emesis
Profound hemorrhage or emesis
 Obstructing lesions (tumor or polyp)
Obstructing lesions (tumor or polyp)
 Trismus
Trismus
 Relative contraindications to cricothyroidotomy are rare, but include:
Relative contraindications to cricothyroidotomy are rare, but include:
 Patients with distortion of neck anatomy due to disease or injury
Patients with distortion of neck anatomy due to disease or injury
 Preexisting laryngeal diseases such as cancer, acute or chronic inflammation, or epiglottitis
Preexisting laryngeal diseases such as cancer, acute or chronic inflammation, or epiglottitis
 Patients who have been intubated translaryngeally for more than 3 days (subglottic stenosis)
Patients who have been intubated translaryngeally for more than 3 days (subglottic stenosis)
 Bleeding diathesis or history of coagulopathy
Bleeding diathesis or history of coagulopathy
 Children less than 10 years of age
Children less than 10 years of age
 There are no absolute contraindications to cricothyroidotomy in adults.
There are no absolute contraindications to cricothyroidotomy in adults.
 Children less than 5 years of age should not undergo cricothyrotomy.
Children less than 5 years of age should not undergo cricothyrotomy.
Complications
 The reported complication rate associated with elective cricothyroidotomy is between 6% and 8% and for emergent procedures, between 10% and 40%.
The reported complication rate associated with elective cricothyroidotomy is between 6% and 8% and for emergent procedures, between 10% and 40%.
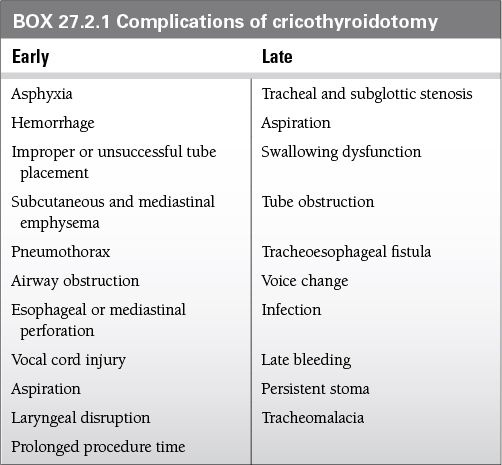
 Adverse effects of cricothyrotomy can be categorized into two groups: those that occur early and those that occur late in the postoperative period (Box 27.2.1).
Adverse effects of cricothyrotomy can be categorized into two groups: those that occur early and those that occur late in the postoperative period (Box 27.2.1).
 Voice change is the most common complication, occurring in up to 50% of cases.
Voice change is the most common complication, occurring in up to 50% of cases.
 Subglottic stenosis is the most frequently reported major complication after cricothyroidotomy.
Subglottic stenosis is the most frequently reported major complication after cricothyroidotomy.
 Complication rate is higher in the pediatric population; pneumothorax is the most common complication in children (5% to 7%).
Complication rate is higher in the pediatric population; pneumothorax is the most common complication in children (5% to 7%).
 The mortality rate in children is up to 8.7%.
The mortality rate in children is up to 8.7%.
Anatomy
 The cricothyroid membrane (ligament) measures 10 mm in height and 22 mm in width and is composed mostly of yellow elastic tissue.
The cricothyroid membrane (ligament) measures 10 mm in height and 22 mm in width and is composed mostly of yellow elastic tissue.
 It covers the cricothyroid space and is located in the anterior neck between the thyroid cartilage superiorly and the cricoid cartilage inferiorly.
It covers the cricothyroid space and is located in the anterior neck between the thyroid cartilage superiorly and the cricoid cartilage inferiorly.
 The cricothyroid space can be easily identified by palpating a slight dip or indentation in the skin immediately below the laryngeal prominence
The cricothyroid space can be easily identified by palpating a slight dip or indentation in the skin immediately below the laryngeal prominence
 It should take less than 5 seconds to identify these landmarks.
It should take less than 5 seconds to identify these landmarks.
 The relative ease of identification, the nearness of the airway to the skin, and the absence of major overlying structures, make cricothyroidotomy the standard emergency surgical airway.
The relative ease of identification, the nearness of the airway to the skin, and the absence of major overlying structures, make cricothyroidotomy the standard emergency surgical airway.
Procedure
 There are several techniques currently used for cricothyrotomy:
There are several techniques currently used for cricothyrotomy:
 Standard technique
Standard technique
 Vertical skin incision followed by horizontal incision through cricothyroid membrane
Vertical skin incision followed by horizontal incision through cricothyroid membrane
 Use of tracheal hook under thyroid cartilage and Trousseau dilator to enlarge cricothyroid membrane incision vertically.
Use of tracheal hook under thyroid cartilage and Trousseau dilator to enlarge cricothyroid membrane incision vertically.
 Insertion of tracheostomy between teeth of Trousseau dilator
Insertion of tracheostomy between teeth of Trousseau dilator
 Rapid four-step technique
Rapid four-step technique
 (1) Palpate cricothyroid membrane; (2) make a horizontal stab incision through skin and cricothyroid membrane; (3) insert tracheal hook and direct inferiorly; (4) insert tracheostomy tube
(1) Palpate cricothyroid membrane; (2) make a horizontal stab incision through skin and cricothyroid membrane; (3) insert tracheal hook and direct inferiorly; (4) insert tracheostomy tube
 Seldinger technique
Seldinger technique
 Catheter over needle is inserted into the trachea through the cricothyroid membrane while aspirating for air.
Catheter over needle is inserted into the trachea through the cricothyroid membrane while aspirating for air.
 Needle is removed leaving catheter tip in trachea.
Needle is removed leaving catheter tip in trachea.
 Guidewire is introduced through catheter, then the catheter is removed.
Guidewire is introduced through catheter, then the catheter is removed.
 A small incision is made in the skin and cricothyroid membrane.
A small incision is made in the skin and cricothyroid membrane.
 The combined tissue dilator-airway catheter unit is advanced over the wire, through the skin incision, and into the trachea.
The combined tissue dilator-airway catheter unit is advanced over the wire, through the skin incision, and into the trachea.
 The dilator and guidewire are removed together, leaving the airway catheter in the trachea.
The dilator and guidewire are removed together, leaving the airway catheter in the trachea.
 Studies on cadavers found that clinicians were 88% successful in performing both the standard technique and the rapid four-step technique, but that the rapid four-step technique was nearly three times faster (43.2 seconds compared with 133 seconds).
Studies on cadavers found that clinicians were 88% successful in performing both the standard technique and the rapid four-step technique, but that the rapid four-step technique was nearly three times faster (43.2 seconds compared with 133 seconds).
 The Seldinger technique appears to lessen the incidence of bleeding and promote a more precise technique of insertion.
The Seldinger technique appears to lessen the incidence of bleeding and promote a more precise technique of insertion.
 Tracheostomy tubes may be easiest to handle, but standard endotracheal tubes (6.0–7.0 for adults) are perfectly acceptable.
Tracheostomy tubes may be easiest to handle, but standard endotracheal tubes (6.0–7.0 for adults) are perfectly acceptable.
 Endotracheal tube position should be confirmed by the same methods as for endotracheal intubation (auscultation, end tidal CO2 monitoring, and chest x-ray).
Endotracheal tube position should be confirmed by the same methods as for endotracheal intubation (auscultation, end tidal CO2 monitoring, and chest x-ray).
Other Considerations
 There are several commercially available cricothyrotomy kits that contain all essential equipment to perform the Seldinger technique.
There are several commercially available cricothyrotomy kits that contain all essential equipment to perform the Seldinger technique.
 Emergent cricothyrotomy is largely a tactile procedure; the field can become bloody after initial incision obscuring the view of any landmarks.
Emergent cricothyrotomy is largely a tactile procedure; the field can become bloody after initial incision obscuring the view of any landmarks.
 Clinicians should be familiar with the relevant anatomy and should be proficient at one of the three commonly used techniques.
Clinicians should be familiar with the relevant anatomy and should be proficient at one of the three commonly used techniques.
 Postprocedure care includes appropriate ventilator settings, sedation, and analgesia.
Postprocedure care includes appropriate ventilator settings, sedation, and analgesia.
 Consultation with surgery or otolaryngology may be necessary with regard to decannulation or conversion to a formal tracheostomy
Consultation with surgery or otolaryngology may be necessary with regard to decannulation or conversion to a formal tracheostomy
SUGGESTED READINGS
Bair AE, Panacek EA, Wisner DH et al. Cricothyrotomy: A 5-year experience at one institution. J Emerg Med. 2003;24(2):151.
Bellhouse CP, Dore C. Criteria for estimating likelihood of difficulty of endotracheal intubation with a Macintosh laryngoscope. Anaesth Intensive Care. 1988;16:329.
Benumof JL. Airway Management Elsevier health sciences: Maryland Heights, MO. Principles and Practice. 1st ed. 1996.
Caparosa RJ, Zavatsky AR. Practical aspects of the cricothyroid space. Laryngoscope. 1957;67:577.
Cole RR, Aguilar EA. Cricothyroidotomy versus tracheostomy: An otolaryngologist’s perspective. Laryngoscope. 1988;98:131.
Cormack RS, Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105.
Glassenburg R, Vaisrub N, Albright G. The incidence of failed intubation in obstetrics: Is there an irreducible minimum abstracted? Anesthesiology. 1990;73:A1061.
Jackson IJB, Choudhry AK, Ryan DW et al. Minitracheostomy: Seldinger – assessment of a new technique. Anesthesia. 1991;46:475.
Kress TD, Balasubramanian S. Cricothyroidotomy. Ann Emerg Med. 1982;11:197.
Lyons G. Failed intubation. Anaesthesia. 1985;40:759.
Samson GLT, Young JRB. Difficult tracheal intubation: A retrospective study. Anaesthesia. 1987;42:487.
Schaumann N, Lorenz V, Schellongowski P et al. Evaluation of Seldinger technique emergency cricothyroidotomy versus standard surgical cricothyroidotomy in 200 cadavers. Anesthesiology. 2005;102(1):7.
Sise MJ, Shackford SR, Cruickshank JC et al. Cricothyroidotomy for long-term tracheal access: A prospective analysis of morbidity and mortality in 76 patients. Ann Surg. 1984;200:13.
Tunstall ME. Failed intubation in the parturient. Can J Anaesth. 1989;36:611 [editorial].
BOX 27.2.1 Complications of cricothyroidotomy
27.3
Bronchoscopy
Jarone Lee
Introduction
 For certification, the American College of Chest Physicians recommends that the bronchoscopist complete the following number of bronchoscopies:
For certification, the American College of Chest Physicians recommends that the bronchoscopist complete the following number of bronchoscopies:
 100 bronchoscopies for initial certification
100 bronchoscopies for initial certification
 25 bronchoscopies per year to maintain certification
25 bronchoscopies per year to maintain certification
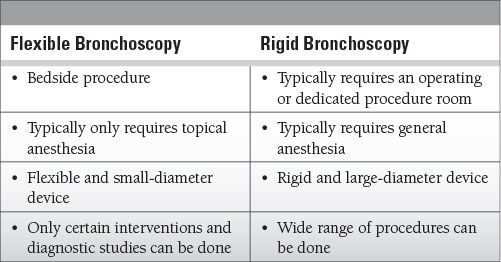
 There are two types of bronchoscopic procedures: flexible and rigid. We focus here on flexible bronchoscopy.
There are two types of bronchoscopic procedures: flexible and rigid. We focus here on flexible bronchoscopy.
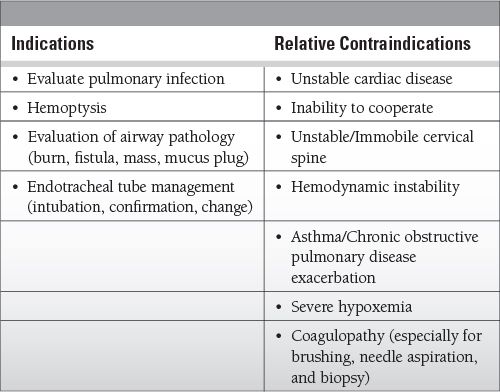
Indications and Contraindications in the ICU
Complications
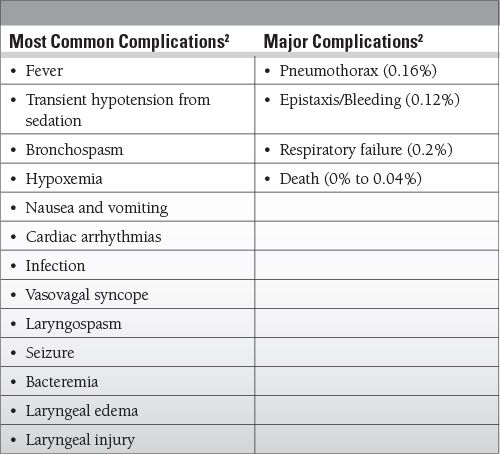
 Complication rate is low: 0.08% to 1.08%
Complication rate is low: 0.08% to 1.08%
 Most complications are from biopsies, especially bleeding and pneumothorax.
Most complications are from biopsies, especially bleeding and pneumothorax.
 Self-limited fever common after bronchoalveolar lavage (BAL) (2%)
Self-limited fever common after bronchoalveolar lavage (BAL) (2%)
Equipment
 The flexible bronchoscope (typically) has the following parts:
The flexible bronchoscope (typically) has the following parts:
 55 to 60 cm scope with 5 to 6 mm diameter
55 to 60 cm scope with 5 to 6 mm diameter
 Light source
Light source
 Image transmission device (fiberoptic or charge-coupled device camera)
Image transmission device (fiberoptic or charge-coupled device camera)
 Instrument insertion channel
Instrument insertion channel
 Suction channel
Suction channel
 Mechanical mechanism to flex and extend distal end of the scope
Mechanical mechanism to flex and extend distal end of the scope
 Other necessary equipment:
Other necessary equipment:
 Topical anesthetic
Topical anesthetic
 Saline flushes
Saline flushes
 Suction
Suction
 Equipment to endotracheally intubate if needed
Equipment to endotracheally intubate if needed
 Cardiac and respiratory monitors
Cardiac and respiratory monitors
 Airway sampling and collection devices
Airway sampling and collection devices
 Bite block for oral approach
Bite block for oral approach
Anatomy
 Classification system: Jackson-Huber, including lobes and segments
Classification system: Jackson-Huber, including lobes and segments
Jackson-Huber Classification
 Right upper lobe (RUL)
Right upper lobe (RUL)
 Apical, posterior, anterior
Apical, posterior, anterior
 Right medial lobe (RML)
Right medial lobe (RML)
 Lateral, medial
Lateral, medial
 Right lateral lobe (RLL)
Right lateral lobe (RLL)
 Superior, medial basal, Anterior basal, lateral basal, Posterior basal
Superior, medial basal, Anterior basal, lateral basal, Posterior basal
 Left upper lobe (LUL)
Left upper lobe (LUL)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree