An integral part of the critical care practice is the administration of blood and blood component therapies. Intensivists must be knowledgeable about the indications, benefits, risks, and adverse effects associated with the use of various blood components.
 Transfusion support is usually required in case of decreased production, increased utilization, destruction, and loss or dysfunction of red cells, platelets, or coagulation factors.
Transfusion support is usually required in case of decreased production, increased utilization, destruction, and loss or dysfunction of red cells, platelets, or coagulation factors.
Available Blood Products
 Red blood cells (RBCs)
Red blood cells (RBCs)
 General information
General information
 Packed red blood cells (PRBCs) are obtained by centrifugation or apheresis (a process where a donor is connected to an automated blood processor, relatively unusual in the United States) methods from whole blood donations.
Packed red blood cells (PRBCs) are obtained by centrifugation or apheresis (a process where a donor is connected to an automated blood processor, relatively unusual in the United States) methods from whole blood donations.
 PRBCs are anticoagulated with citrate and are generally mixed with a preservative solution that increases the storage period to up to 42 days at 1 to 6°C.
PRBCs are anticoagulated with citrate and are generally mixed with a preservative solution that increases the storage period to up to 42 days at 1 to 6°C.
 One unit of compatible RBCs, which contains approximately 250 to 300 mL of volume with a hematocrit of 55 to 65%, will increase the hemoglobin (Hgb) level by approximately 1 g/dL or hematocrit by 3% in an average sized, non-bleeding/hemolyzing adult.
One unit of compatible RBCs, which contains approximately 250 to 300 mL of volume with a hematocrit of 55 to 65%, will increase the hemoglobin (Hgb) level by approximately 1 g/dL or hematocrit by 3% in an average sized, non-bleeding/hemolyzing adult.
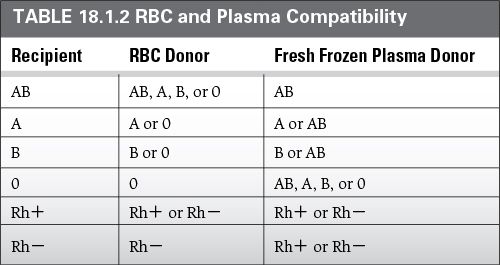
 Donor RBCs must be either ABO identical or compatible (refer to Table 18.1.2).
Donor RBCs must be either ABO identical or compatible (refer to Table 18.1.2).
 Indications
Indications
 RBCs are indicated in symptomatic, anemic patients to restore oxygen carrying capacity.
RBCs are indicated in symptomatic, anemic patients to restore oxygen carrying capacity.
 Hypovolemia of any cause without a significant reduction in cell mass should be managed with crystalloid/colloid solutions.
Hypovolemia of any cause without a significant reduction in cell mass should be managed with crystalloid/colloid solutions.
 In the setting of massive bleeding (>1 to 2 L/hour), RBCs should be considered as a replacement solution.
In the setting of massive bleeding (>1 to 2 L/hour), RBCs should be considered as a replacement solution.
 While optimal threshold for transfusion is not established, the use of Hgb levels alone is discouraged.
While optimal threshold for transfusion is not established, the use of Hgb levels alone is discouraged.
 The decision to transfuse or not should be based on the clinical situation (volume status, shock or hypoperfusion, duration and severity of anemia, and cardiopulmonary status).
The decision to transfuse or not should be based on the clinical situation (volume status, shock or hypoperfusion, duration and severity of anemia, and cardiopulmonary status).
 In hemodynamically stable patients, a lower Hgb trigger for transfusion (transfuse if Hgb < 7) has proved to be as effective as a higher Hgb trigger (Hb < 10).
In hemodynamically stable patients, a lower Hgb trigger for transfusion (transfuse if Hgb < 7) has proved to be as effective as a higher Hgb trigger (Hb < 10).
 Special considerations
Special considerations
 Leukocyte-reduced red cells (LRRCs) can be prepared by different filtration techniques in the blood blank but can also be accomplished at the bedside.
Leukocyte-reduced red cells (LRRCs) can be prepared by different filtration techniques in the blood blank but can also be accomplished at the bedside.
 LRRCs are primarily indicated in the following situations:
LRRCs are primarily indicated in the following situations:
 Prevention of non-hemolytic febrile transfusion reaction
Prevention of non-hemolytic febrile transfusion reaction
 Prevention of human leukocyte antigens (HLA) immunization (possible worse post-transfusion platelet increase)
Prevention of human leukocyte antigens (HLA) immunization (possible worse post-transfusion platelet increase)
 Prevention of cytomegalovirus (CMV) in organ transplant recipients
Prevention of cytomegalovirus (CMV) in organ transplant recipients
 High-risk populations (e.g., cardiac surgery) with systemic endothelial dysfunction related to the systemic inflammatory response
High-risk populations (e.g., cardiac surgery) with systemic endothelial dysfunction related to the systemic inflammatory response
 Washed red cells are useful in preventing severe allergic transfusion reactions mediated by recipient antibodies (IgE) to donor soluble proteins.
Washed red cells are useful in preventing severe allergic transfusion reactions mediated by recipient antibodies (IgE) to donor soluble proteins.
 The isotonic saline washing process virtually removes all the donor’s plasma proteins.
The isotonic saline washing process virtually removes all the donor’s plasma proteins.
 Washing blood products can introduce bacteria into the product.
Washing blood products can introduce bacteria into the product.
 Washed cells must be transfused within 24 hours if kept at 1 to 6°C.
Washed cells must be transfused within 24 hours if kept at 1 to 6°C.
 Frozen red cells are cryo-protected with glycerol and can be stored for more than 10 years with good viability.
Frozen red cells are cryo-protected with glycerol and can be stored for more than 10 years with good viability.
 Once thawed and prepared, they must be transfused within 24 hours.
Once thawed and prepared, they must be transfused within 24 hours.
 This can be useful in military setting and for rare phenotypes.
This can be useful in military setting and for rare phenotypes.
 Retrospective studies suggest that transfusing blood stored for a prolonged period of time (>14 days of storage) may be associated with increased morbidity; however, no prospective data have confirmed such results.
Retrospective studies suggest that transfusing blood stored for a prolonged period of time (>14 days of storage) may be associated with increased morbidity; however, no prospective data have confirmed such results.
 Platelets (PLTs)
Platelets (PLTs)
 General information
General information
 Also referred to as whole blood-derived platelets (WB-PLTs) or single donor platelets (SDPs)
Also referred to as whole blood-derived platelets (WB-PLTs) or single donor platelets (SDPs)
 WB-PLTs (50 mL) are prepared by centrifugation of individual whole blood units.
WB-PLTs (50 mL) are prepared by centrifugation of individual whole blood units.
 Typically four to six WB-PLTs are pooled prior to transfusion to an adult recipient.
Typically four to six WB-PLTs are pooled prior to transfusion to an adult recipient.
 SDPs are collected from one single donor using various apheresis devices.
SDPs are collected from one single donor using various apheresis devices.
 Both preparations are stored at room temperature (20 to 24°C) and are licensed for a maximum of 5 days of storage.
Both preparations are stored at room temperature (20 to 24°C) and are licensed for a maximum of 5 days of storage.
 They contain an appreciable volume of plasma.
They contain an appreciable volume of plasma.
 Approximately 30,000 to 60,000/mm3 increase in the recipient’s platelet count is expected for each SDP or pool of 6 WB-PLT administered.
Approximately 30,000 to 60,000/mm3 increase in the recipient’s platelet count is expected for each SDP or pool of 6 WB-PLT administered.
 Apheresis PLTs and pooled WB-PLTs have the same hemostatic efficacy.
Apheresis PLTs and pooled WB-PLTs have the same hemostatic efficacy.
 Indications
Indications
 PLTs should be administered to correct a decrease in platelet count (thrombocytopenia) or function (thrombocytopathy).
PLTs should be administered to correct a decrease in platelet count (thrombocytopenia) or function (thrombocytopathy).
 Given normal PLT function, the target count depends upon the indication for PLT transfusion (prophylactic or therapeutic).
Given normal PLT function, the target count depends upon the indication for PLT transfusion (prophylactic or therapeutic).
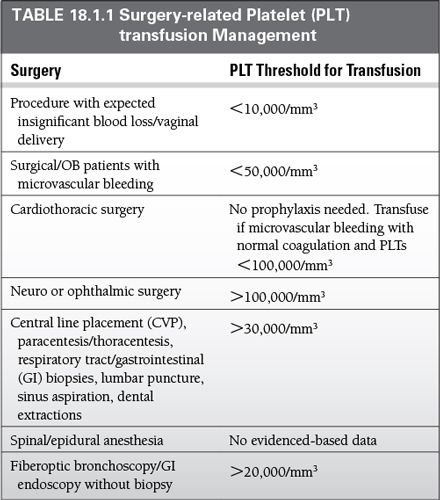
 PLTs should be maintained at >50,000/mm3, for the therapeutic treatment of active bleeding, thrombocytopenia, and disorder of PLT function.
PLTs should be maintained at >50,000/mm3, for the therapeutic treatment of active bleeding, thrombocytopenia, and disorder of PLT function.
 PLTs should be maintained at >100,000/ mm3 for central nervous system or multisystem trauma.
PLTs should be maintained at >100,000/ mm3 for central nervous system or multisystem trauma.
 For prophylactic purposes, and to prevent spontaneous hemorrhage, PLTs should be transfused with PLTs of <10,000/mL.
For prophylactic purposes, and to prevent spontaneous hemorrhage, PLTs should be transfused with PLTs of <10,000/mL.
 In the setting of heparin-induced thrombocytopenia, thrombotic thrombocytopenia purpura and hemolytic-uremic syndrome, PLT transfusion should be avoided unless in the case of life-threatening bleeding.
In the setting of heparin-induced thrombocytopenia, thrombotic thrombocytopenia purpura and hemolytic-uremic syndrome, PLT transfusion should be avoided unless in the case of life-threatening bleeding.
 ABO matching is not strictly necessary, but it is preferred with neonates and small volume pediatric patients who can develop hemolysis from passive transfer of anti-A/anti-B from the donor plasma.
ABO matching is not strictly necessary, but it is preferred with neonates and small volume pediatric patients who can develop hemolysis from passive transfer of anti-A/anti-B from the donor plasma.
 PLT-related transfusion management decisions should always take into consideration the presence of circulating antiplatelet medications (ASA, clopidogrel, GP IIb/IIIa antagonists, etc.), herbal compounds (garlic, Danshen, etc.), alloantibodies, and disorders with known effects on platelet functions like uremia, hypersplenism, disseminated intravascular coagulopathy (DIC), and sepsis.
PLT-related transfusion management decisions should always take into consideration the presence of circulating antiplatelet medications (ASA, clopidogrel, GP IIb/IIIa antagonists, etc.), herbal compounds (garlic, Danshen, etc.), alloantibodies, and disorders with known effects on platelet functions like uremia, hypersplenism, disseminated intravascular coagulopathy (DIC), and sepsis.
 For surgery-related PLT management, see Table 18.1.1.
For surgery-related PLT management, see Table 18.1.1.
 Plasma products
Plasma products
 Fresh frozen plasma (FFP)
Fresh frozen plasma (FFP)
 General information
General information
 Plasma is the remaining part of the whole blood after removal of platelets and cellular elements.
Plasma is the remaining part of the whole blood after removal of platelets and cellular elements.
 The product is generally frozen within 8 hours of phlebotomy to prevent inactivation of factors V and VIII, which are temperature-sensitive.
The product is generally frozen within 8 hours of phlebotomy to prevent inactivation of factors V and VIII, which are temperature-sensitive.
 At a temperature lower than −18°C, FFP can be stored for up to 1 year.
At a temperature lower than −18°C, FFP can be stored for up to 1 year.
 Before transfusion, the product must be thawed in a water bath at 37°C for approximately 30 minutes. Then transfusion must occur within 24 hours.
Before transfusion, the product must be thawed in a water bath at 37°C for approximately 30 minutes. Then transfusion must occur within 24 hours.
 If not thawed, plasma can be stored for an additional 4 days at 1 to 6°C.
If not thawed, plasma can be stored for an additional 4 days at 1 to 6°C.
 Factor V and VIII fall to 80% and 60% of normal in this case.
Factor V and VIII fall to 80% and 60% of normal in this case.
 Indications
Indications
 Use FFP for the treatment of micro-vascular bleeding due to congenital or acquired coagulopathies resulting in prolongation of either The partial thromboplastin time (PTT) or activated partial thromboplastin time (aPTT or APTT) greater than 1.5 times normal.
Use FFP for the treatment of micro-vascular bleeding due to congenital or acquired coagulopathies resulting in prolongation of either The partial thromboplastin time (PTT) or activated partial thromboplastin time (aPTT or APTT) greater than 1.5 times normal.
 In emergent situations, use FFP in combination with intravenous vitamin K to reverse the effect of warfarin prior to surgery or during active bleeding.
In emergent situations, use FFP in combination with intravenous vitamin K to reverse the effect of warfarin prior to surgery or during active bleeding.
 Special considerations
Special considerations
 In general, sufficient hemostasis can be achieved when the activity of coagulation factors is at least 25 to 30% of normal and the level of fibrinogen is at least 75 to 100 mg/dL.
In general, sufficient hemostasis can be achieved when the activity of coagulation factors is at least 25 to 30% of normal and the level of fibrinogen is at least 75 to 100 mg/dL.
 Therefore, for an average adult, a dose of approximately 10 to 15 mL/kg (3 to 5 units) FFP.
Therefore, for an average adult, a dose of approximately 10 to 15 mL/kg (3 to 5 units) FFP.
 Donor plasma must be either ABO-identical or ABO-compatible with the recipient (Table 18.1.2).
Donor plasma must be either ABO-identical or ABO-compatible with the recipient (Table 18.1.2).
 Volume loading should be considered.
Volume loading should be considered.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Special considerations
Special considerations