Definition: study of the absorption, distribution, metabolism, and excretion of drugs in the body
 Absorption
Absorption
 Drugs administered extravascularly must be absorbed across biologic membranes to reach the systemic circulation.
Drugs administered extravascularly must be absorbed across biologic membranes to reach the systemic circulation.
 Extent and rate of drug absorption are determined by physiochemical properties, formulation, and route of administration.
Extent and rate of drug absorption are determined by physiochemical properties, formulation, and route of administration.
 Non-intravenous administration may result in reduced absorption.
Non-intravenous administration may result in reduced absorption.
 Enteral absorption may be reduced due to gut hypomotility, hypoperfusion, changes in gastrointestinal (GI) pH, and enteral feeding.
Enteral absorption may be reduced due to gut hypomotility, hypoperfusion, changes in gastrointestinal (GI) pH, and enteral feeding.
 Subcutaneous/intramuscular/transdermal absorption may be reduced due to hypoperfusion of muscles, skin, and splanchnic organs.
Subcutaneous/intramuscular/transdermal absorption may be reduced due to hypoperfusion of muscles, skin, and splanchnic organs.
 Bioavailability (F)—the fraction of unchanged drug that reaches the systemic circulation following administration
Bioavailability (F)—the fraction of unchanged drug that reaches the systemic circulation following administration
 Drugs given intravenously have 100% bioavailability.
Drugs given intravenously have 100% bioavailability.
 Drugs administered orally have reduced bioavailability primarily due to incomplete absorption and first-pass elimination.
Drugs administered orally have reduced bioavailability primarily due to incomplete absorption and first-pass elimination.
 First-Pass Effect—drug removed from the blood or plasma via GI and hepatic enzymes following absorption from the GI tract, prior to reaching the systemic circulation
First-Pass Effect—drug removed from the blood or plasma via GI and hepatic enzymes following absorption from the GI tract, prior to reaching the systemic circulation
 Results in a significant reduction in bioavailability
Results in a significant reduction in bioavailability
 Drugs that undergo first-pass effect will require dose reductions when given intravenously.
Drugs that undergo first-pass effect will require dose reductions when given intravenously.
 Examples: diltiazem, labetolol, lidocaine, metoprolol, morphine, nitroglycerin, verapamil, propranolol
Examples: diltiazem, labetolol, lidocaine, metoprolol, morphine, nitroglycerin, verapamil, propranolol
 Distribution
Distribution
 The passing of drug molecules from the blood into tissues and organs
The passing of drug molecules from the blood into tissues and organs
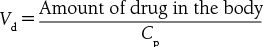
 Vd is affected by drug permeability, protein binding, tissue binding, and organ perfusion
Vd is affected by drug permeability, protein binding, tissue binding, and organ perfusion
 Table 17.1.1 describes the pharmacokinetic factors that influence Cp and Vd
Table 17.1.1 describes the pharmacokinetic factors that influence Cp and Vd
 Drugs tend to have an increased Cp if they have low lipid solubility, increased plasma protein binding, or decreased tissue binding and as a result will have a reduced Vd.
Drugs tend to have an increased Cp if they have low lipid solubility, increased plasma protein binding, or decreased tissue binding and as a result will have a reduced Vd.
 Metabolism
Metabolism
 The chemical conversion of a drug molecule into a metabolite, typically via an enzymatic reaction
The chemical conversion of a drug molecule into a metabolite, typically via an enzymatic reaction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


