1. The undertreatment of pain in children remains a problem. This is because pain and pain management are difficult problems made more complex by the ever changing physical and psychological states seen in children as they mature.
2. Multiple methodologies and medications can be combined to provide a framework for the treatment of pediatric pain, beginning by identifying and targeting the mechanisms of pain in an individual patient. Tailoring therapeutic interventions to that individual’s needs based on neurophysiologic status and pharmacogenetic profile, rather than employing empiric treatments aimed just at symptom management, should enhance the efficacy and efficiency of pediatric pain management.
3. The analgesic plan should be considered and initiated before any painful interventions are to occur. In the case of surgery, this should include the preoperative administration of analgesics and pain relieving adjuncts in conjunction with the institution of cognitive, behavioral, and complementary therapies as an effective way of preempting pain.
4. To avoid untoward events, careful attention to drug dosing, pharmacogenetic class, and medical status is necessary in children.
5. The use of regional blockade and other adjunctive therapies can reduce the incidence of both inadequate analgesia and associated opioid side effects.
THE INTERNATIONAL ASSOCIATION FOR THE STUDY OF PAIN (IASP) defines pains as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage” (1). Such an expansive definition of pain emphasizes the subjective nature of pain and how psychosocial, developmental, ethnic, genetic, and cultural factors impact its experience. Defining pain in this manner gives credence to the pain experienced by a fearful child awaiting a vaccination or intravenous cannulation, as well as an infant undergoing a circumcision. Furthermore, pain can occur and exist in the absence of tissue damage. A caregiver is urged to address both the psychological and physical aspects of pain in order to effectively and reliably treat it (2,3). Table 10.1 lists many of the pain terms mentioned in this chapter and their definitions.
Pain is a protective sensation that acts as an early warning system designed to minimize tissue damage. This is the positive aspect of pain (4). On the other end of the spectrum, negative characteristics have both an immediate impact on the well-being of a child and may result in long-term detrimental consequences (5,6). The latter is the result of prolonged stimulation and pathological alterations to the peripheral or central nervous system (CNS) brought about by untreated or inadequately managed pain (7,8,9). Children may suffer mild to severe pain from a vast array of encounters during the perioperative period (10-12). Most often, this type of pain is characterized as somatic pain. It is describer as acute, well localized, and sharp in nature. Children also encounter episodes of visceral or neuropathic pain. Visceral pain is characterized as diffuse and aching, whereas neuropathic or dysesthetic pain associated with nerve damage is burning in nature. Acute pain from minor and major procedures is associated with acute illness or an acute exacerbation of a chronic illness such as in sickle cell anemia or cancer.
Chronic pain also occurs in children (7,13). Migraines, cancer pain, inflammatory bowel disease, and phantom limb pain all contribute to childhood suffering and disability. In spite of the known presence of pain in children, epidemiological studies demonstrate the continued lack of adequate management in this population (14,15). Gaining an understanding of the pathophysiology of pain and how to apply age-appropriate pain assessment tools will help create targeted pain treatment plans that promote the implementation of successful pain management strategies and improved pain control in children (6,16).
TABLE 10.1 Pain terms
Allodynia—lowered threshold: pain due to a stimulus that does not normally provoke pain
Analgesia—absence of pain in response to stimulation which would normally be painful
Anesthesia dolorosa—pain in an area or region which is anesthetic
Causalgia—a syndrome of sustained burning pain, allodynia, and hyperpathia after a traumatic nerve lesion, often combined with vasomotor and sudomotor dysfunction and later trophic changes
Central pain—pain initiated or caused by a primary lesion or dysfunction in the CNS
Dysesthesia—an unpleasant abnormal sensation, whether spontaneous or evoked
Hyperalgesia—an increased response to a stimulus which is normally painful
Hypoalgesia—diminished pain in response to a normally painful stimulus
Hyperpathia—a painful syndrome wherein a nociceptive stimulus evokes an exaggerated pain response
Hypoesthesia—decreased sensitivity to stimulation, excluding the special senses
Neuropathic pain—pain initiated or caused by a primary lesion or dysfunction in the nervous system
Neuralgia—pain in the distribution of a nerve or nerves
A sample list of frequently used terms from Merskey H, Bogduk N. IASP Task Force on Taxonomy: Classification of Chronic Pain. 2nd ed. Seattle, WA: IASP Press; 1994:209–214.
I. Neuroscience of pain
A. Transduction, transmission, perception, and modulation are processes that define pain. Pain is distinguished from nociception in that pain is a perception, while nociception is the biophysical process that encodes noxious stimuli that often, but not always, leads to the expression of pain (17).
B. Several types of pain are produced by noxious mechanical, thermal, or chemical injury—nociceptive pain, inflammatory pain, and neuropathic pain (18).
1. Specialized pain receptors, nociceptors, on the afferent somatosensory A-δ and C neurons transduce noxious stimuli into electrical activity. These encoded nociceptive signals are transmitted through sensory neurons traveling through the dorsal horn of the spinal cord.
2. Sensory neurons synapse on secondary neurons that cross the midline of the spinal cord and transmit the nociceptive signals, usually through specific ascending “pain tracts” that include the spinothalamic, spinoparabrachial, spinomesencephalic, and spinoreticular tracts.
3. The signals from the periphery project into a “neuromatrix” that includes the thalamus, hypothalamus, anterior cingulate cortex, somatosensory cortex brain stem, periaqueductal gray (PAG) brain stem reticular (nuclei), and locus ceruleus. These tracts spread to a wide variety of sites in the brain that are involved with nociceptive processing (see Fig. 10.1).
C. Signals reaching the somatosensory cortex are perceived as pain. Other signals project into the midbrain and areas that are involved with the affective components of pain (19).
D. Pain modulation occurs through neuronal projections from the PAG area and the nucleus raphe magnus, which form descending pathways that inhibit or facilitate pain signals at lower levels of the CNS, including the substantia gelatinosa (the Rexed lamina II) (20).
E. Current research indicates that the neuroanatomic and neuroendocrine systems necessary to perceive pain are present by the 25th week of gestation, but the descending inhibitory systems are not completely developed until sometime after birth. Fetuses greater than 24 weeks gestational age and neonates are therefore more likely to have an increased sensitivity to painful stimuli (also see Chapter 1) (21,22).
F. Tissue injury produces a neuroendocrine stress response and inflammatory immunologic changes that modulate pain (16,23). Local inflammatory reaction promulgated by mast cells, macrophages, and neutrophils produces leakage of plasma, increased capillary wall permeability, and the release of mediators—kinins, amines, arachidonic acid derivatives, tumor necrosis factor (TNF), purines, potassium ions, hydrogen ions, serotonin, primary afferent amines, proteases, and nerve growth factor.

FIGURE 10.1 Cortical and subcortical projections of pain processing pathways. (Adapted from Morgan GE, Mikhail MS, Murray MJ. Clinical Anesthesiology. 4th ed. New York, NY: McGraw-Hill; 2006:363.)
1. The inflammatory reaction is one process that decreases the threshold for neuronal activation, and results in hyperalgesia, leading to chronic pain.
2. Intracellular translational and transcriptional changes alter the properties of the pain neurons such that pain cells are more excitable and react to innocuous stimuli, producing central sensitization or “wind-up” (24,25). With central sensitization, pain becomes self-sustaining and very difficult to treat (26). Even nonnoxious sensations can evoke pain if wind-up is present; examples of which are phantom limb pain, complex regional pain syndromes type 1 (CRPS-1), migraine headaches, allodynia, fibromyalgia, and myofascial pain syndrome (27–30).
II. Pain assessment. Assessment of its impact on the patient is the most important first step to managing pain (31). Multiple pain assessment instruments or measures exist. Choosing the correct age-appropriate and situationally aware instrument can be difficult because many patients are nonverbal or are not cognitively aware. Knowledge of the properties and limitations of available assessment instruments provides a means of choosing the correct one (32).
A. Pain assessment tools may be classified as:
1. Self-report (visual analog or verbal numeric scales)
2. Behavioral (graded behavioral activities such as crying or change in limb activity) (Table 10.2)
3. Physiological (parameter such as heart rate and blood pressure are scored)
4. Biochemical (neuroendocrine responses are targeted)
5. Neurophysiological (electromyogram [EMG], near-infrared spectroscopy [NIRS], and electroencephalogram [EEG])
B. Good pain measurement tools must have (a) validity, (b) reliability, and (c) minimal bias.
1. To be valid, pain measure must measure a specific aspect of a child’s pain (e.g., intensity) so that changes in a child’s pain rating represent a meaningful and proportional change in the child’s pain experience.
2. The measure must provide trustworthy and consistent pain ratings that do not change over time.
3. The measure must be free from response bias, that is, an instrument that will not direct the respondent to a particular answer.
4. In addition, the pain measure should be practical. If the measurement is too cumbersome, it will not be functional.
5. Finally, it is critical to note that a pain scale is one tool of several that facilitates clinical decision making. It should not be used in isolation to assign a specific intervention to a predetermined score (33).
C. Self-report is often the most accurate method for assessing pain. This type of tool relies on the child’s cognitive abilities to understand what is being asked and to integrate that into an expression of what they are feeling, and thus its use is limited to children who possess the requisite cognitive and expressive capacities. Since the subjective nature of the pain experience must account for the patient’s response to a stimulus, self-reporting scales are able to incorporate this parameter better than other methods. By removing the subjective nature of observational pain assessment, appropriate pain assessment instruments can be a factor in preventing treatment disparities based on race (15,34–36). An example of a self-report tool can be found in Figure 10.2 (37).
1. Problems with this class of assessment tools include failure to account for the influence of the interrogator and desire to please the questioner by giving the “correct answer.”
2. Children, like adults, also deny pain as a means of avoiding painful interventions such as examinations or injections.
3. Some children and adolescents may have difficulty in objectively rating their pain. Others, depending on underlying personality traits, may tend to overrate or underrate their pain for various reasons, including secondary gain. Since pain is a multifaceted experience whose components are qualitative, it may be very difficult to compare one child’s quantitative assessment of pain with another’s (38,39). Not everyone’s 8 out of 10 pain is the same. The only comparable level between subjects might be zero pain.
D. Although there exists a wide variation in the accuracy of parental estimation of pediatric pain, this input remains an invaluable tool in one’s assessment and management of the pediatric patient (40).
1. If the child is verbal, age-appropriate questions with the help of visual aids may prompt discussion regarding the perioperative course of events. This in turn allows the practitioner the opportunity to address fears and misconceptions the patient and/or parents may have.
2. Real or imagined threats of bodily mutilation, parental separation, pain, or death are often translated into immediate or long-term behavioral problems. Having this discussion early may be effective in reducing stress and thereby decrease the pain experience as well as any potential long-term behavioral sequelae.
3. For the preverbal child, neonates, and infants, physiologic and behavioral assessment tools work the best when evaluating the severity of pain (41). Blood pressure, heart rate, oxygen saturation, pupil size, and palmar sweating all denote pain in the preverbal child.
E. Neonates, premature infants, infants, and nonverbal patients represent a population in which pain assessment is a difficult task. For the younger of these patients, the lack of nervous system maturity and dearth of learned behavioral responses and language deficiency present a challenging situation for pain assessment (42).
1. Physiologic pain measures in conjunction with carefully applied behavioral scales have proven to be the best tools for measuring pain in this group.
2. Because multiple other nonpain factors impacting these younger patients can cause physiologic changes, assessment tools relying on these parameters alone may have limited accuracy.
3. Neurophysiologic parameters are being tested as another, possibly more accurate, measure of pain (43). Table 10.3 lists several pain assessment tools commonly used in pediatrics (44).

FIGURE 10.2 Faces Pain Scale. (Redrawn from Hicks CL, von Baeyer CL, Spafford P, et al. The Faces Pain Scale—Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183.)
CLINICAL PEARL When using behavioral pain scales, be sure to score each parameter; do not give a composite score based on a “gestalt” impression of the child’s overall appearance. The score is not valid unless each metric is independently assessed.
III. Pain prevention—nonpharmacologic interventions. Pain prevention is an important first step in controlling pain. Both nonpharmacologic and pharmacologic treatment have proven efficacy in children. The importance of the psychological state and complexity of a patient’s personality and their psychosocial background cannot be overstated (i.e., the importance cannot be overstated).
A. The social and psychological composition of the child and their family dynamics influences pain sensitivity and therapeutic efficiency in multiple ways (45).
B. Memories of previous pain experiences and socialization around painful events all influence subsequent reactions to pain (46).
C. The increasing trend toward the use of multimodal and interdisciplinary approaches to pain management in children has provided more tools to effectively treat and possibly preempt pain (47). A full discussion of the patient’s pain problems and the use of complementary therapies should take place as early as possible in a child’s hospitalization so that the therapies are viewed as a component of the therapeutic pain plan as opposed to a last ditch effort at controlling pain. Table 10.4 contains a limited list of the nonpharmacologic interventions that can have a significant role in the multimodal treatment plans for children.
1. Nonpharmacologic interventions are represented by a number of cognitive and behavioral techniques that include mind-body therapies such as play therapy, hypnosis, imagery, and distraction.
2. Other therapies such as acupuncture, acupressure, and massage are classified as manipulative therapies. If available, these also can be recommended to patients to assist in coping with pain (48).
3. Therapies such as acupressure and acupuncture are useful for both pain and symptom management (49). Anxiety, irritability, and gastrointestina (GI) problems have all been effectively treated with this ancient therapy.
TABLE 10.4 Mind-body interventions
Mind-body therapies (cognitive behavioral therapies [CBT]) | Manipulative therapies |
Biofeedback | Acupuncture |
Meditation | Acupressure |
Distraction including virtual reality, play therapy | Reiki |
Imagery | Watsu |
Thought stopping | Swaddling |
Psychotherapy | Heat and cold stimulation |
Music | Transcutaneous electrical nerve stimulation (TENS) |
Hypnosis | Exercise including yoga |
Desensitization | Desensitization |
4. Hypnosis is effective for some patients, producing significant positive effects on their ability to cope with and modulate pain (50).
D. It is important to ensure that any therapy is age appropriate and not contraindicated based on the child’s medical condition, cultural, or religious beliefs. Enlisting parental assistance, nursing staff help, or any of the other myriad of available caretakers including child life therapists, psychologists, physical therapists, and complementary medicine practitioners who are skilled in the different nonpharmacologic interventions can provide support during painful or anxiety-provoking situations. Remember that these therapies are employed as adjuncts and may not relieve all pain by themselves. Choose the therapy to match the child’s circumstances (51–53).
IV. Acute pain management—pharmacologic interventions. Mistaken beliefs about the value of pain in promoting the ideal of “manhood,” the fear of addiction, trepidation about overdosing, and the untoward side effects of pain medications combined with a lack of knowledge about pediatric pharmacology often lead to dosing errors of pain medications in the pediatric population. This is especially true in the postoperative period.
• In the pursuit of a safer, more effective pain management strategy, a graded, multimodal approach is advocated. This approach employs a number of different strategies, including a variety of drugs that have different mechanisms of action such that pain is targeted through different pathways.
• This strategy allows a physician to deliver less toxic amounts of an individual agent because of the sometimes synergistic effects of combining agents (54-57). The addition of nonopioid adjuvants has been shown to reduce the odds of serious adverse opioid events in the postoperative period (58). As well, the magnitude of the intervention is commensurate with the level of pain the patient is experiencing.
• Knowledge of the molecular mechanisms of a drug and of its pharmacodynamic effects will improve therapy.
A. Opioids. Opioids represent a broad class of agents that have excellent analgesic properties. They are used for mild to severe pain and are often easily titratable and available. As the cornerstone of most pain treatment regiments, a working knowledge of the opioid pharmacology is appropriate. Focusing on both peripheral and central molecular targets of this class of drug along with newer functional radiological imagery has facilitated a deep insight into the actions of these agents (59).
• Opioids target a restricted set of receptors in the nervous system. They bind µ, κ, δ, and the nociceptin opioid receptors (NOP)/orphanin FQ peptide receptor on primary sensory neurons.
• There are multiple subgroups of each type of opioid receptor, which sometimes demonstrate subtle difference in their responses (60).
• These receptors are located in nerve cells in the spinal cord, basal ganglia, amygdala, rostral ventral medulla, and PAG regions of the brain. Opioid receptors are also found on cardiovascular endothelial cells and in the membranes of some immune cells (61).
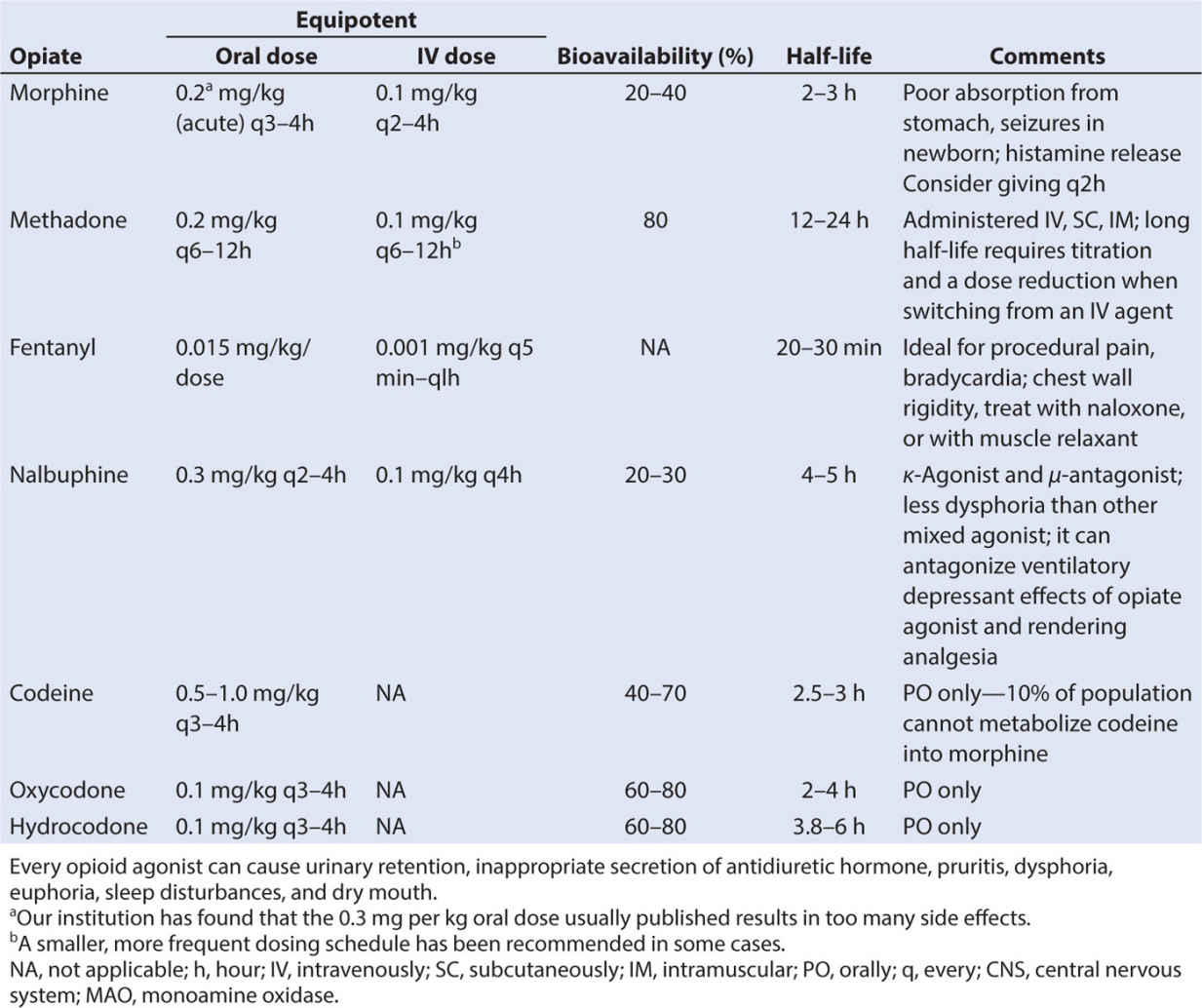
• There are three classes of opioids that bind to opioid receptors: naturally occurring (morphine and codeine), semisynthetic (hydromorphone and oxycodone), and synthetic (fentanyl, alfentanil, sufentanil, methadone, and meperidine) (62). See Table 10.5.
• Opioids are also classified by their action as full agonists, partial agonists, and mixed agonists antagonists (63).
• When opioids bind to their receptors, specific G-proteins located on presynaptic and postsynaptic neurons that are coupled to G-proteins are activated. Activation causes a cascade of events: inhibition of adenyl cyclase and inhibition of the release of glutamate, substance P, and calcitonin.
• Opioid binding on presynaptic neurons promotes suppression of calcium channels, and binding on postsynaptic neurons suppresses potassium efflux. This results in membrane hyperpolarization and subsequent inhibition of nerve transmission along the pain neurons expressing opioid receptors (61,64).
• Depending on their preparation, opioids can be administered by multiple routes: orally, intramuscularly, subcutaneously, rectally, intrathecally, epidurally, sublingually, intranasally, and transdermally.
CLINICAL PEARL It is important to note that the absorption and bioavailability of any drug via disparate routes may be dramatically different, and may thereby alter an agent’s analgesic efficacy.
• Analgesic potency of several opioids is found in Table 10.6, along with IV dosing recommendations in Table 10.7. Potency of other agents is often judged relative to morphine since it is considered the gold standard analgesic. Starting with weak opioids and progressing to very strong agents, the choice of opioid will depend on a number of factors, including the side effect profile of the agent, the route of administration, presence of allergies and sensitivities, or sensitivities, and the analgesic needs of the child.
• Several caveats about the opioids should be noted. When switching or converting to another opioid drug, even when using a conversion table properly, enhanced sedation and respiratory depression can occur, especially in patients who are receiving chronic opioids. This phenomenon has been attributed to “incomplete tolerance.” Reducing the calculated dose by one-fourth to one-third and administering breakthrough bolus doses of pain medication can minimize untoward effects. The total daily administered dose of pain medication can be adjusted by evenly distributing the total dose as boluses throughout the day. Oral dosing and equivalency conversions from IV dosing can be found in Table 10.8.
• Side effects common to all opioids include respiratory depression, bronchoconstriction, myoclonus, chest wall rigidity, pruritus, depression of the immune system, vomiting, nausea, constipation, sedation, hallucinations, dysphoria, tolerance, urinary retention, and hyperalgesia. These adverse drug events can become so pronounced that some patients would rather experience pain than tolerate the opioid side effects. Monitoring and using preemptive or prophylactic therapy are extremely important and should be included in any treatment protocols. Patients can be encouraged with the knowledge that most side effects decrease over time, the only exception to this being constipation.
1. Codeine is a naturally occurring prodrug that is classified as a phenanthrene. Given orally (0.5 to 1 mg/kg PO every 4 hours) alone or with acetaminophen, it can be administered for mild pain.
a. To be effective, codeine must be demethylated by the liver, converting it to the active metabolite morphine. CYP2D6 is the cytochrome P450 isoform responsible for the conversion.
b. Approximately 10% of children lack one or more copies of the cytochrome P450 isoenzymes involved in codeine metabolism. These children will therefore receive little to no analgesia from codeine (65).
c. There is another segment of the population that is classified as ultrarapid metabolizers. These people have more than two copies of the wild-type alleles for CYP2D6. They metabolize codeine faster than normal, potentially producing toxic blood levels of morphine. The deaths of several children have been caused by ultrametabolism of codeine. In one well-publicized case, a breast-fed infant died whose mother was prescribed codeine for tonsillectomy pain and was an ultrarapid metabolizer (66,67).
d. If a child is determined to be an ultrarapid metabolizer, codeine administration must be avoided, and because the polymorphism is not commonly tested for, it is probably best to avoid the use of codeine in favor of other oral opioid analgesics.
2. Oxycodone is a strong, semisynthetic thebaine compound that is manufactured in multiple oral forms, including a sustained release formulation (Oxycontin, Purdue Pharmaceuticals, Stamford, CT). It is used for moderate to severe pain. The immediate release product is dosed 0.1 to 0.15 mg per kg orally every 4 hours. The sustained release product is administered every 12 to 24 hours, and is reserved for persistent long-term pain.
a. Oxycodone is converted in the liver by the CYP450 enzymes to multiple metabolites, including oxymorphone.
b. The half-life in children is shorter than morphine, and it has a high bioavailability (68). As with codeine, there are ultrarapid metabolizers that have lower pain relief from normal doses of the drug. Patients who are poor metabolizers are at increased risk for toxicity from oxycodone since they are likely to consume more doses than recommended to try to achieve analgesia. The addictive potential of the sustained release form has recently been highlighted.
CLINICAL PEARL Despite the hepatic conversion of oxycodone via CYP450 isoenzymes to other pharmacologically active compounds, the clinical impact appears to be significantly less with this drug compared with codeine. It has come into favor as a codeine replacement in pediatrics and is available as a titratable liquid formulation.
3. Morphine is a benzylisoquinoline agent classified as a phenanthrene. It is the “gold standard” against which all other pain control methodologies are measured and is often the first-line therapy for severe acute pain. Derived from the poppy seed, morphine binds to µ-opioid receptors to create its positive and negative actions. It is metabolized extensively in the liver but also in the brain and kidneys.
a. There are two primary active metabolites, morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). M6G has properties similar to morphine, that is, respiratory depression and analgesia. Its potency in humans is variable depending on the model in which it is tested (69).
(1) The quantity of each of these two metabolites present in plasma and urine changes with age. Each decreases as the metabolic capabilities increase.
(2) Because these products are metabolized by the liver and excreted by the kidneys, impairment can result in accumulation of these metabolites and may be responsible for side effects such as respiratory depression, increased analgesia, increased hyperalgesia, and a lower seizure threshold (70).
(3) In infants, elimination is more dependent on renal function, because their glucuronidation pathways are limited and the drug undergoes a greater proportion of sulfation, with urinary excretion of the sulfated compound.
(4) M3G has antianalgesic or antagonist properties at the opioid receptors, so if too much M3G is produced, analgesia will be reduced. Patients on chronic morphine therapy who become resistant or tolerant should have their dose reduced.
(5) M3G is neuroexcitatory and proconvulsant. The mechanism is not fully elucidated, but may act through the gabaergic/glycerine pathways (71).
(6) Morphine causes vasodilation, pruritus, respiratory depression, nausea, vomiting, constipation, an ileus, sedation, delirium, and addiction.
CLINICAL PEARL Pruritus from systemically administered morphine is in part due to histamine release, but there are also central spinal mechanisms for this unpleasant side effect. While antihistamine drugs can be used for treatment, low doses of opioid antagonists or mixed agonist–antagonists may be particularly helpful while avoiding additional sedation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree