Fig. 11.1
Causes of opioid-induced nausea and/or vomiting
Since tolerance to opioid-induced nausea and vomiting usually develops within days, prolonged treatment, if any, is usually not needed. Often, decreasing the opioid dose with a slower titration to escalating doses, changing the route of administration, or opioid rotation are sufficient strategies to manage this side effect. If possible, identifying an individual’s trigger of opioid-induced nausea and/or vomiting can allow one to tailor etiology-specific treatment. If symptoms occur with movement or ambulation, the vestibular system may be involved and treatment with antihistamines or anticholinergics such as scopolamine may be of benefit. If symptoms are associated with meals, gastrointestinal causes may be triggering nausea and vomiting and the patient may benefit from multiple smaller volume meals as well as treatment with a motility agent such as metoclopramide [3].
If a clear mechanism cannot be identified, treatment is often determined based on a patient’s comorbidities and other symptoms such as constipation. The potential for side effects from the treating medication must also be considered. Other potentially therapeutic drugs include prokinetic drugs, antipsychotics and related drugs, antihistamines, serotonin antagonists, anticholinergics, benzodiazepines, and steroids (see Table 11.1). Prokinetic agents such as metoclopramide improve gastric motility and decrease GI transit times and, therefore, may be beneficial to patients with nausea and constipation [4]. Antipsychotics work within the chemoreceptor trigger zone to block dopamine receptors and have been found to be effective for the treatment of opioid-associated nausea and vomiting in cancer patients [5]. Antihistamines act on the emesis center and vestibular system [3, 6]. Serotonin antagonists act by blocking serotonin release in the GI tract and the chemoreceptor trigger zone [6]. Anticholinergics act on the emesis center and GI tract [6]. Benzodiazepines act on the vestibular system and chemoreceptor trigger zone. The mechanism of steroids in reducing nausea and vomiting is not clear [6]. Peripherally acting mu-receptor antagonists have been shown to decrease gastric transit time and diminish opioid-related nausea and vomiting [7, 8] but have not been extensively used for this purpose. Medications may be delivered in a non-oral form if nausea and vomiting impair oral intake or if oral intake is limited for other reasons. A combination of antiemetics from different classes may be needed in resistant cases. All of these medications may result in side effects of their own, and the risks and benefits of initiating these therapies as opposed to decreasing the opioid dose, changing the route of administration, changing the type of opioid, or discontinuing opioid therapy must be considered.
Motility drugs | Metoclopramide |
Antipsychotics and related drugs | Haloperidol, droperidol, prochlorperazine, promethazine, chlorpromazine, methotrimeprazine |
Antihistamines | Meclizine, cyclizine, hydroxyzine, diphenhydramine |
Serotonin antagonists | Ondansetron, granisetron, dolasetron |
Anticholinergics | Scopolamine |
Benzodiazepines | Lorazepam |
Steroids | Prednisone, dexamethasone |
Potentially, peripherally acting mu-receptor antagonist | Methylnaltrexone, alvimopan |
Additionally, some have recommended use of alternative therapies including cannabinoids and acupressure [9]. Cannabinoids have been primarily studied for the treatment of nausea and vomiting related to chemotherapy. The results of the studies in this setting have shown some efficacy in patients who have failed treatment with other antiemetics but often resulted in a high rate of therapy discontinuation secondary to adverse side effects [10]. Cannabinoids are not commonly used in the setting of opioid-induced nausea and vomiting and are not recommended for routine use in this setting. Acupuncture techniques including acupressure and electro-acutherapy treatments with use of acupuncture point Pericardium 6 have been found to be effective in the treatment of postoperative nausea and vomiting [11] as well as in a multitude of other settings including chemotherapy-induced nausea and vomiting [12]. Other alternative approaches include use of meditation and guided imagery to treat the anticipation of nausea and vomiting and have been studied in patients with cancer [13]. There is not a large amount of data supporting use of these treatments for opioid-induced nausea and vomiting, but they may be beneficial.
Most importantly, if an opioid has not recently been started or opioid dose recently escalated, or if there are other systemic signs and symptoms, other etiologies of nausea and/or vomiting must be considered (see Table 11.2).
Gastrointestinal (GI) | Functional (e.g., irritable bowel syndrome, dyspepsia, gastroparesis) |
Obstruction (e.g., adhesions, malignancy, hernia, stenosis) | |
Organic (e.g., appendicitis, cholecystitis, hepatitis, peptic ulcers) | |
Infectious | (e.g., Food-borne toxins, urinary tract infection) |
Medications/toxins | (e.g., Nonsteroidal anti-inflammatories, radiation) |
Metabolic | (e.g., Adrenal disorder, pregnancy, uremia) |
Miscellaneous | (e.g., Nephrolithiasis, pain, psychiatric) |
Central nervous system | Increased intracranial pressure, migraine, seizure disorder, head injury, vestibular |
Constipation
Constipation is a common side effect of opioid therapy. Constipation related to opioid use has been reported to occur in 15–90 % of patients [15]. Constipation as defined by the Rome III Diagnostic Criteria includes at least two of the following: less than three defecations per week, straining, lumpy or hard stools, feeling of incomplete evacuation or anorectal obstruction, and manual attempts to ease defecation [16].
There are multiple potential mechanisms for opioid-induced constipation, and it is widely known to be mediated via opioid receptors in the GI tract and central nervous system (see Table 11.3). Binding of opioid receptors, specifically mu, kappa, and delta receptors, within the enteric nervous system of the GI tract alters GI motility and contributes to constipation [17–19] as well as to nausea and vomiting. Once bound, the release of GI neurotransmitters is inhibited disrupting the appropriate intestinal contractions needed for GI motility, and mucosal secretions are decreased [15].
Constipation is thought to be dose-related, and unfortunately, tolerance to opioid-induced constipation does not typically occur [7]. Therefore, this side effect usually necessitates treatment. Lifestyle and dietary modifications including increasing physical activity, drinking more fluids, ingesting larger amounts of fiber, and creating a meal and toileting schedule may be trialed [20]. Typically, first-line treatment includes use of a stool softener such as sodium docusate and often is not effective as a sole treatment [21]. A stimulant laxative such as senna is typically added to use of stool softener. If constipation persists, addition of osmotic or bulk-forming laxatives, nonabsorbable solution, or enema may be therapeutic (see Table 11.4). Recently, peripherally acting mu-receptor antagonists have been found effective in treating peripheral causes of constipation. Methylnaltrexone is approved for treatment of opioid-induced constipation in patients receiving palliative care, and alvimopan is approved for aiding GI function after bowel resection [8].
Stool softeners | Sodium docusate |
Stimulants | Senna, bisacodyl |
Osmotic laxatives | Sorbitol, lactulose, magnesium, polyethylene glycol |
Bulk laxatives | Wheat bran, psyllium seed, polycarbophil |
Lubricant | Mineral oil |
Peripherally, acting mu-receptor antagonist | Methylnaltrexone, alvimopan |
As with most opioid side effects, one may consider decreasing opioid dose, opioid rotation, changing the route of administration, or discontinuing opioid therapy. There is literature reporting diminished rates of constipation with use of buprenorphine or transdermal fentanyl, but there is also literature refuting this in regard to transdermal fentanyl [23–25]. If opioid therapy has not recently been initiated or opioid dose increased, or if there are other symptoms, one must consider other causes of constipation (see Table 11.5).
Functional | Dietary reasons, motility problem |
Structural | Anorectal disorders, colonic strictures, or mass lesions |
Endocrine and metabolic | Diabetes mellitus, hypercalcemia, hypothyroidism |
Neurogenic | Cerebrovascular events, multiple sclerosis |
Smooth muscle and connective tissue disorders | Amyloidosis, scleroderma |
Psychogenic | Anxiety, depression, somatization |
Drugs | Narcotics, anticholinergics, antidepressants |
Neuroendocrine Effects Including Hypogonadism
Opioids are known to alter the functioning of the hypothalamus-pituitary-adrenal axis in both acute and chronic settings. The hypothalamus has numerous functions including controlling the secretion of gonadotropin-releasing hormone (GrH), which stimulates the pituitary gland to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). These hormones, then, act on the testes and ovaries to result in the secretion of testosterone and estradiol. The release of GrH, LH, and FSH are regulated by negative feedback from testosterone and estradiol (see Fig. 11.2).
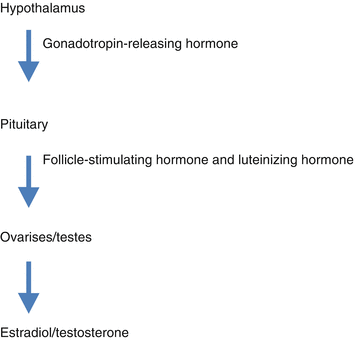

Fig. 11.2
The hypothalamus-pituitary axis
Chronic opioid therapy leads to suppression of the hypothalamus and pituitary [27–30]. This results in diminished GrH secretion contributing to a decreased release of LH and FSH as well as decreased testosterone and estradiol. Studies support the finding of opioid receptors in the hypothalamus and pituitary as well as the gonads [27, 28, 30–33]. Therefore, opioid-induced hypogonadism is thought to be mediated via central effects on the hypothalamus and pituitary and via direct gonadal effects.
Opioids also have been shown to alter neuroendocrine function by increasing thyroid-stimulating hormone and prolactin while decreasing oxytocin [34]. Additionally, studies have shown opioids may impact the autonomic nervous system and result in altered glycemic control and insulin release [34].
Opioid suppression of the hypothalamus, pituitary, and gonads can result in hypogonadism in both males and females potentially leading to fatigue, depression, anxiety, decreased libido, infertility, decreased muscle mass, osteopenia, osteoporosis, and compression fractures in either sex (see Table 11.6) [29, 35]. In females, there may be amenorrhea and oligomenorrhea and, in males, erectile dysfunction. Daniell reported that 87% of men receiving opioids noted a decreased libido or significant erectile dysfunction after initiating use of opioids [27]. This study was followed by a study treating men with opioid-induced androgen deficiency with testosterone and revealed that testosterone treatment resulted in improved sexual function, decreased depression, and improved mood [36].
Table 11.6
Symptoms of hypogonadism
In females | Amenorrhea, oligomenorrhea |
In males | Erectile dysfunction |
In both sexes | Decreased libido, decreased fertility, decreased muscle mass, osteopenia, osteoporosis, compression fractures, fatigue, decreased ability to concentrate, depression, anxiety |
Patients receiving chronic opioid therapy with symptoms of hypogonadism should be monitored for abnormalities in sex hormones. Laboratory analysis should include free and total testosterone and estradiol in women. It has also been recommended that LH and dehydroepiandrosterone, an adrenal precursor to the primary sex hormones, be tested as well [37, 38]. If diagnosed with hypogonadism secondary to opioids, it has been suggested that the risks and benefits of continued opioid therapy be considered versus discontinuation of opioids, a trial of alternative opioid, or hormonal supplementation [37]. In current practice, sex hormones are often monitored if the patient has complaints consistent with hypogonadism as outlined above while receiving chronic opioid therapy. Hormonal supplementation may be beyond the scope of some pain management practitioners and require coordinated care through the patient’s primary care physician or with referral to endocrinology. Nonetheless, sex hormone levels should be monitored in all symptomatic patients and potentially in all patients receiving chronic opioid therapy. In addition to monitoring of free testosterone, estradiol, and other hormones, measuring bone density may also be valuable [29]. The American Association of Clinical Endocrinologists recommends ordering bone density studies for all patients with hypogonadism [35]. Furthermore, depending on the patient’s history, other causes of hypogonadism should be considered as well (see Tables 11.7 and 11.8). Many of these would likely have presented earlier in life or with additional symptoms. Detailed evaluation of these conditions may be left to an endocrinologist or other specialist.
Pituitary tumors, pituitary insufficiency, hemochromatosis |
Hyperprolactinemia |
Transient hypogonadism due to serious illness or stress |
Aging, metabolic syndrome |
Autoimmune syndromes, acquired immunodeficiency syndrome |
Fertile eunuch syndrome, mumps, orchitis |
Cryptorchidism, vanishing testes syndrome, testicular trauma |
Radiation treatment or chemotherapy |
Sertoli cell only syndrome |
Genetic syndromes: |
Klinefelter’s syndrome, 47 XYY syndrome, dysgenetic testes |
Androgen receptor defects, testicular feminization, Reifenstein’s syndrome |
5 Alpha-reductase deficiency, myotonic dystrophy, Kallmann’s syndrome |
Reproductive organ dysfunction | Congenital, Asherman’s syndrome, Turner’s syndrome, premature ovarian failure including secondary to autoimmune causes, polycystic ovarian syndrome, ovarian tumors |
Pituitary disorders | Tumor, hemochromatosis, sarcoidosis, traumatic brain injury |
Hypothalamic dysfunction | Kallmann’s syndrome, radiation |
Prolactin secreting tumors | |
Hypercortisolism | |
Thyroid disorders |
Immune Effects
Opioids, especially morphine, are known to adversely affect the immune system. Morphine has been shown to inhibit phagocytosis as early as 1898 [41]. Numerous subsequent studies have verified this including early animal research by Kraft and Leitch in 1921 demonstrating a decreased resistance to streptococcal septicemia in animals treated with morphine [42].
This immunosuppression occurs via both central and direct cellular mechanisms. Centrally, opioids can lead to the release of corticosteroids via the hypothalamic-pituitary-adrenal axis [43], leading to suppression of immune system function with chronic use of opioids [44]. Acute exposure to morphine and related drugs is also believed to influence the immune system via activation of the sympathetic nervous system [43, 44]. The released catecholamines, including norepinephrine, bind to leukocytes to alter immune function [45]. Opioids can also directly affect immune cells via opioid receptors present on immune cells [43]. Studies suggest that opioids bind to immune cell opioid receptors resulting in a diminished ability of immune cells to produce lymphocytes and macrophage precursors [43]. Further studies reveal that immune cells possess mu-opioid receptors and are activated by morphine, which is the most immunosuppressive opioid [44].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





