Fig. 1.1
Schematic displays the defining psychophysical properties that characterize the pain report after an acute high-intensity stimulus (left) and that after a local tissue injury (right). As indicated, in the inset plotting pain score vs. stimulus intensity, with the acute stimulus, the pain report for a given displays a threshold above which there is a monotonic increase in the magnitude of the reported pain state. After a tissue injury, there is an ongoing pain, and a stimulus applied to the injury site reveals that there is a greater pain report for a given stimulus (e.g., a primary hyperalgesia). In addition, it is appreciated that an innocuous mechanical stimuli applied to an adjacent uninjured area yields an enhanced response referred to secondary tactile allodynia
Tissue Injury
The psychophysics of pain associated with a tissue injury and inflammation has several distinct psychophysical elements that distinguish it from the events initiated by an acute high-intensity stimulus (Fig. 1.1):
(i)
With local tissue injury (such as burn, abrasion, or incision or the generation of a focal inflammatory state as in the joint), an acute sensation is generated by the injuring stimulus which is followed by an ongoing dull throbbing aching sensation which typically referred to the injury site—skin, soft tissue, or joint—and which evolves as the local inflammatory state progresses.
(ii)
Application of a thermal or mechanical stimulus to the injury sited will initiate a pain state wherein the pain sensation is reported to be more intense than would be expected when that stimulus was applied to a non-injured site. That is to say, as shown in Fig. 1.1, the stimulus response curve is shifted up (e.g., an ongoing pain) and to the left. This lowered threshold of stimulus intensity required to elicit an aversive response to a stimuli applied to the injury site is referred to as primary hyperalgesia. Thus, modest flexion of an inflamed joint or moderate distention of the gastrointestinal (GI) track will lead to behavioral reports of pain.
(iii)
Local injury and a low-intensity stimuli applied to regions adjacent to the injury may also produce a pain condition, and this is referred to as 2° hyperalgesia or allodynia. Thus, light touch may be reported as being aversive and is referred to as tactile allodynia.
These examples of “sensitization” secondary to local injury and inflammation are observed in all organ systems. Common examples would be sunburn (skin inflammation) leads to extreme sensitivity to warm water, inflammation of the pleura leads to pain secondary to respiration, and eyelid closure is painful secondary to corneal abrasion [1, 2].
In the case of inflammation of the viscera, the ongoing pain sensations are typically referred to specific somatic dermatomes. Thus, cardiac ischemia is referred to the left arm and shoulder, while inflammation of the bowel is associated with ongoing pain and hypersensitivity to light touch applied to the various quadrants of the abdomen. Such “referred” pain states reflect the convergence of somatic and visceral pain systems [3].
Nerve Injury
As described by Silas Weir Mitchell in 1864, frank trauma leads to two identifying elements: ongoing dysesthetic pain typically referred to the dermatome innervated by the injured nerve and prominent increase in the sensitivity to light touch applied to these regions. Injury to the nerve may be initiated by a wide variety of physical (extruded intervertebral disc compression section), toxic (chemotherapy), viral (postherpetic neuralgia: HIV), and metabolic (diabetes). In most of these syndromes, these two elements are expressed to varying degrees [4].
Encoding of Acute Nociception
As outlined in Fig. 1.2, the systems underlying these effects of acute high-intensity stimulation may be considered in terms of the afferents, the dorsal horn, and projection components. Under normal conditions, activity in sensory afferents is largely absent. However, peripheral mechanical and thermal stimuli will evoke intensity-dependent increases in firing rates of lightly myelinated (A∂) or unmyelinated (C) afferents. Based on differential blockade, these two fiber types, differing markedly in conduction velocity, are thought to underlie the acute sharp pain and subsequent dull throbbing sensation, respectively.
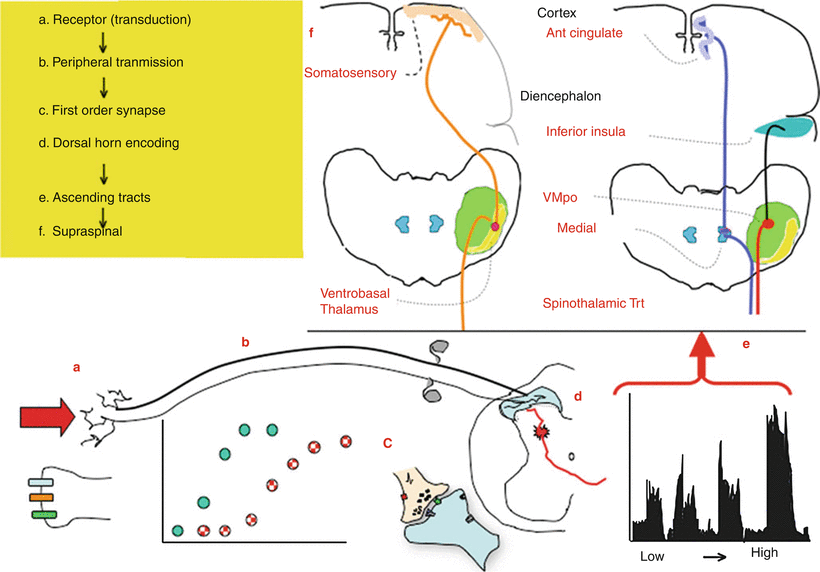

Fig. 1.2
This schematic provides an overview of the organization of events that initiate pain state after an acute high-intensity stimulus applied to the skin. (a) A physical stimulus activates channels such as the TRP channels on the terminal of small diameter afferents (light line). (b) There are two classes of afferents: large low-threshold afferents (Ab: dark line) and small high-threshold afferents (A∂/C: light line). As the stimulus intensity increases, there is a monotonic increase in the discharge rate of each class of afferents with the low-threshold afferents showing an increase at low intensities, whereas the high-intensity afferents show an increase at higher intensities. The low and high threshold afferents project respectively into the deep and superficial dorsal horn. (c) The afferent input leads to depolarization of these spinal afferent terminals (d) which release excitatory transmitters yielding an (e) intensity-dependent depolarization of the second-order neuron. (Shown here is an example of the response of a neuron receiving convergent input from high and low threshold afferents.) Populations of these neurons project into the contralateral ventrolateral pathways to project to higher centers. (f) Broadly speaking, there are two classes of outflow. There are those which project into the somatosensory (ventrobasal) thalamus which then sends projection to the somatosensory cortex. A second type of projections goes to more medial thalamic regions and sends projection into areas of the old limbic forebrain (e.g., anterior cingulate/inferior insula)
Transduction Channels and Afferent Terminal Activation
The transduction of the physical stimulus is mediated by specific channels which increase their conductance when certain stimulus properties are present. Channels vary in the range of temperatures which activate them, ranging from hot (such as the TRPV1) to cool to cold (TRPM1). The acute response properties of the afferent are thus defined by the collection of transducer channels that are expressed on its terminals. Activation of these channels increases inward sodium and calcium currents and progressive depolarization of the terminal (Fig. 1.2a) [5, 6].
Chemical Sensitivity of Temperature Channels
An important element regarding these channels is that while they are to varying degrees temperature sensitive, they also show sensitivity to specific chemicals. Thus, the TRPV1 responds to capsaicin, while the TRPM1 responds to menthol. Accordingly, when these agents are applied to the tongue, the sensation associated with their application corresponds with the sensation produced by the fibers which normally activate these fibers, hot and cool, respectively [7].
Action Potential Generation
Peripheral terminal depolarization leads to activation of the voltage-gated sodium channels which then leads to action potentials in the respective afferent. Subtypes of sodium channels (designated as NaV 1.1 through NaV 1.9 channels) have been identified.
These channels differ in terms of their activation properties as well as their pharmacology (e.g., tetrodotoxin sensitive or insensitive) and their distribution. Thus, some channels may be found principally on unmyelinated afferents (Nav 1.7) or distributed widely on all types of excitable membranes ranging from myocytes to brain neurons to a variety of afferents. Importantly, the frequency of afferent discharge is proportional to terminal depolarization which is proportional to stimulus intensity (Fig. 1.2b) [8, 9].
Encoding Properties of Primary Afferents
There are three important properties that define the encoding properties of any given class of primary afferents (Fig. 1.2b) [10]:
(i)
Under resting conditions, the primary afferent, whether A or C, shows little or no spontaneous activity.
(ii)
Primary afferents typically begin to respond to their respective stimulus modality (e.g., Aβ-tactile or C-thermal/mechanical/chemical) at some minimal intensity (e.g., threshold).
(iii)
Above threshold, the frequency of firing evoked in the afferent axon will be proportional to stimulus intensity over a range of intensities. “Low-threshold” afferents will typically discharge at intensities that are considered to be nonnoxious. “High-threshold” or nociceptive afferents will discharge at intensities that are considered to be aversive in character.
Afferent Synaptic Transmission
Afferent action potentials invade the spinal terminal and depolarize these terminals. Such activation opens voltage-sensitive calcium channels which activate a variety of synaptic proteins which mediate the mobilization of synaptic vesicles and thereby initiate transmitter release.
Calcium Channels and Afferent Transmitter Release
There are a variety of voltage-sensitive calcium channels that regulate terminal transmitter release (referred to as CaV channels). These channels are distinguished on the basis of their voltage sensitivity, their location, and the agents which block them. The best known of these channels are the N-type calcium channel blocked by the therapeutic agent ziconotide. Block of this channel will block the release of many afferent terminal transmitters [11].
Spinal Afferent Terminal Transmitters
Sensory afferent uniformly releases excitatory transmitters. In terms of transmitters (Table 1.1), virtually all afferents contain and release the excitatory amino acid glutamate. Small afferent releases not only glutamate but also one or more of several peptides, such as substance P or calcitonin gene-related peptide (CGRP) (Fig. 1.2c). These transmitters in turn act postsynaptically upon eponymous receptors present on several of populations of dorsal horn neurons (Fig. 1.2d) [12]:
Table 1.1
Overview of classes of primary afferents characterized by common transmitter and cell markers
Fiber | Spinal termination | Cell marker | Channel/receptor | Transmitter | |||
|---|---|---|---|---|---|---|---|
NF200 | IB4 | TRPV1 channel | Mu opioid receptor | Glutamate | Peptide (sP) | ||
Aβ | III–VI | X | X | ||||
A∂ | II, III–V1 | X | X | ||||
C | I–II | X | X | X | X | X | |
C | II | X | |||||
(i)
Glutamate exerts the primary depolarizing effect through the activation of the AMPA receptor which leads to a short lasting increase in sodium conductance yielding a potent and short lasting depolarization of the membrane.
(ii)
Substance P acts upon a G protein-coupled receptor that leads to a long slow depolarization of the membrane. Importantly, such receptors lead not only to a depolarization of the membrane, but to an increase in intracellular calcium, the glutamate by activating voltage-sensitive calcium channel and the NK1 by mobilizing intracellular calcium stores.
Laminar Organization of Spinal Dorsal Horn
The spinal dorsal horn is organized transversely in laminae (Rexed laminae), ranging from the most superficial dorsal horn marginal layer (lamina I), the substantia gelatinosa (lamina II), and the deeper nucleus proprius (laminae III–VI) [13].
Primary Afferent Projections
There are several important principles reflecting the pattern of termination of afferent terminals in the dorsal horn (Fig. 1.3) [14]:
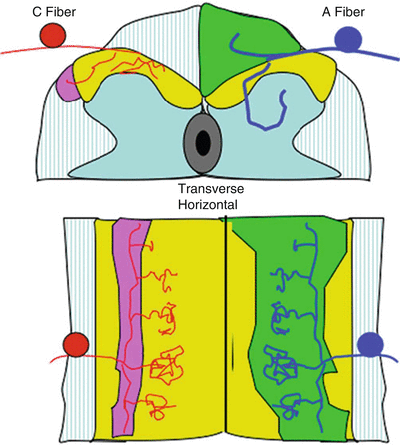

Fig. 1.3
Schematic presenting the spinal cord in transverse section (top) and horizontal (bottom) and showing: (i) (left) the ramification of C fibers in the superficial dorsal horn (laminae I and II) and collateralization into the tract of Lissauer and (ii) (right) the ramification of A fibers in the dorsal horn (terminating in the deep dorsal horn) and collateralization rostrocaudally into the dorsal columns and at each segment into the dorsal horn. The densest terminations are within the segment of entry. There are less dense collateralizations into the dorsal horns at the more distal spinal segments. This density of collateralization corresponds to the potency of the excitatory drive into these distal segments. Thus, distal segments may receive input from a given segment, but the input is not sufficiently robust to initiate activation of the neurons in the distal segment under normal circumstances
(i)
Small afferents (A∂/C) terminate superficially in the lamina I (marginal layer) and lamina II. In contrast, the large afferents (Aβ) project deep into the dorsal horn and curve upwards to terminate just deep to lamina III.
(ii)
Observing the spinal cord from the dorsal surface, it is noted that the central processes of the afferents collateralize, sending processes rostrally and caudally up to several segments into the dorsal columns (large afferents) or in the tract of Lissauer (small C-fiber afferents). Periodically, these collaterals send sprays into the dorsal horn at distal segments. Thus, neurons up to several segments distal to a given root entry zone of any given segment will receive afferent input from a given root (e.g., the L5 root will make synaptic contact with dorsal horn cells as far rostral as spinal segment L1). Importantly, the primary excitation occurs at the level of entry where the synaptic connections are strongest. At more distal segments, the degree of excitation from the proximal root is progressively reduced.
Dorsal Horn Neurons
Based on the organization of afferent termination, one can appreciate that superficial lamina I marginal neurons are primarily activated by small, high-threshold afferent input; hence they are “nociceptive specific.” In contrast, the deeper lying cells have their cell bodies in lamina V and are hence called lamina V neurons but send their dendrites up into the superficial laminae. Interestingly, they receive input from Aβ (low-threshold) input on their ascending dendrites and C-fiber (high-threshold) input on their distal terminals (Fig. 1.4). Accordingly, these cells with their convergent input show activation at low intensities (mediated by the Aβ input) and increasing activation as the intensity rise (mediated by the C-fiber input). Accordingly, as shown in Fig. 1.2e, the cell shows increasing discharge rates over the range from very low to very high-threshold stimuli. Accordingly, these cells are referred to as wide-dynamic-range (WDR) neurons [15].
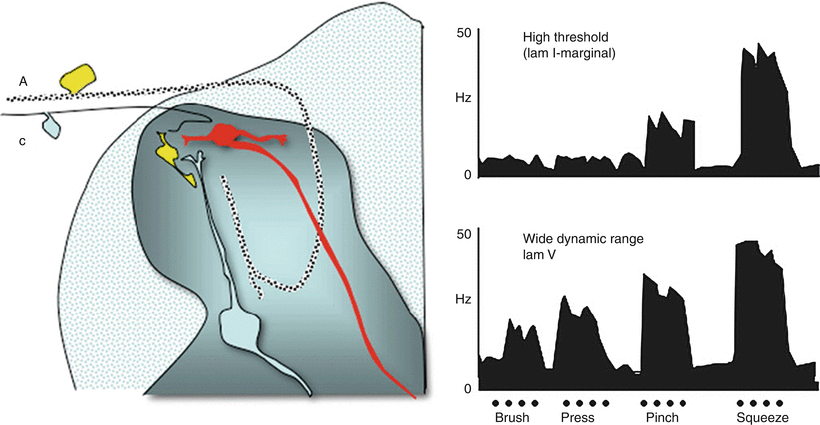

Fig. 1.4
Schematic displays (left) two principal classes of second-order neurons. As indicated, small afferents tend to terminate superficially (laminae I and II), while large afferents tend to project deep into the dorsal horn and terminate below lamina II. Accordingly, cells lying in lamina I (marginal layer) receive largely high-threshold input. Cells lying deeper (lamina V) received input from large afferents on their proximal dendrites and can receive excitatory input directly or through excitatory interneurons on their distal dendrites. (right) Single-unit recording from spinal dorsal horn, showing firing pattern (impulses/s) of a (top) high-threshold (nociceptive-specific marginal) neuron and (bottom) a horn wide-dynamic-range neuron, (WDR) located primarily in lamina V in response to graded intensities of mechanical stimulation (brush, pressure, pinch, squeeze) applied to the receptive fields of each cell. Both cells project supraspinally. Note the relationship between firing patterns and the response properties of the afferents with which each cell makes contact
Dorsal Horn Projections
These lamina I and lamina V neurons then project via the ventrolateral tracts to higher centers and thence to cortical levels. Projections may occur ipsilaterally or contralaterally in the ventrolateral tracts. Ipsilaterally projecting axons typically project to terminate in the medial brainstem reticular nuclei. Cells receiving these projections then project to the thalamus. Contralateral axons project into several thalamic nuclei [13, 16].
Supraspinal Organization
The supraspinal projections can be broadly classified in two motifs (Fig. 1.2e) [13]:
(i)
Dorsal horn, ventrobasal thalamic complex-somatosensory cortex. This is the classic somatosensory pathway. In these cases, the nervous system undertakes to maintain a specific intensity-, spatial-, and modality-linked encoding of the somatic stimulus, as summarized in Fig. 1.2. This pathway possesses the characteristics that relate to the psychophysical report of pain sensation in humans and the vigor of the escape response in animals. In the absence of tissue injury, removal of the stimulus leads to a rapid abatement of the afferent input and disappearance of the pain sensation. At all levels, the intensity of the message is reflected by the specific populations of axons which are activated and by the frequency of depolarization: the more intense the stimulation, the more frequent is the firing of the afferent; the greater is the dorsal horn transmitter release, the greater is the evoked discharge and the higher is the frequency of firing in the ascending pathway.
(ii)
Dorsal horn-medial thalamus-limbic cortex. Here, there appears to be little precise anatomical mapping. Cells in this region project to regions such as the anterior cingulate cortex or inferior insula. The anterior cingulate is part of the older limbic cortex and is believed to be associated with emotional content.
The above subdivision reflects the orthogonal component of the pain experience, notably the “sensory-discriminative” components (“I hurt here on a scale of 1–10, 6”) and the “affective-motivational” component of the pain pathway (“I have cancer, I am mortal”) as proposed by Ronald Melzack and Kenneth Casey.
Encoding of Nociception After Tissue Injury
As reviewed above, with tissue injury, a distinct pattern of aversive sensations is observed. The psychophysical profile noted with injury or inflammation is composed of (i) an ongoing sensory experience that is described as dull throbbing aching ongoing pain, (ii) enhanced responsiveness to subsequent stimulation (e.g., hyperalgesia/tactile allodynia), and (iii) secondary pain referral (e.g., sensations which are aversive when applied to adjacent uninjured areas).
Peripheral Changes in Afferent Transmission Resulting from Tissue
As described in Fig. 1.1, in the event that a stimulus leads to a local injury, as in a tissue crush (trauma) or an incision, such stimuli may lead to the subsequent local elaboration of active products that directly activate the local terminals of afferents (that are otherwise silent) innervating the injury region and facilitating their discharge in response to otherwise submaximal stimuli. This then leads to an ongoing afferent barrage and enhanced response to any given stimulus (e.g., peripheral sensitization) (Fig. 1.5).
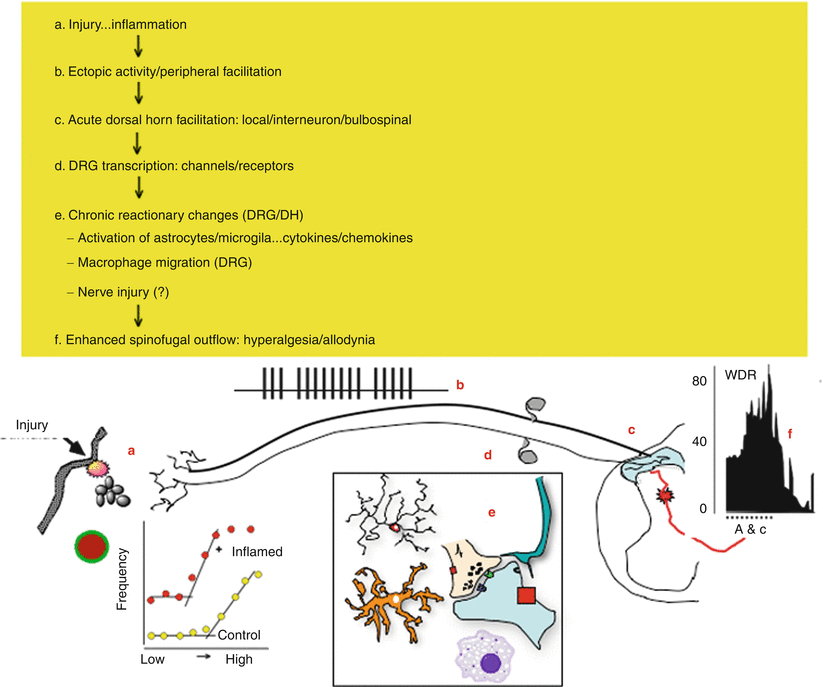

Fig. 1.5
This schematic provides an overview of the organization of events that initiate pain state after a tissue injuring stimulus of the skin. (a) Local tissue injury leads to the initiation of an innate immune response that yields the release of a variety of active factors. The factors acting through eponymous receptors on the terminals of C fibers lead to an activation of the C fiber and a state of sensitization. Accordingly, such products initiate an ongoing activity and an enhanced response to an otherwise innocuous stimulus. (b) The injury thus leads to an ongoing activity in small afferent. (c) The ongoing activity activates dorsal horn neurons and initiates a state of facilitation (windup). (d) The ongoing afferent traffic and injury products lead to a change in the tropic functions of the dorsal root ganglion leading to changes in protein synthesis and the expression of various receptors and channels which serve to enhance afferent responsiveness. (e) In the dorsal horn, the ongoing afferent drive initiates additional changes related to the activation of microglia and astrocytes as well as the invasion of typically nonneuronal cells including neutrophils and lymphocytes in the extreme. The net effect is to enhance the outflow initiated by any given stimulus, for example, hyperalgesia and allodynia. (f) With facilitation, the wide dynamic range neurons (WDR) are activated in response to stimuli that would normally not activate these neurons.[AW1]
Origin of Ongoing Activity and Enhanced Terminal Responsiveness After Tissue Injury
The source of these active factors may be considered in terms of their source including the following (Fig. 1.5):
(i)
Damaged cells which yield increased extracellular contents (potassium).
(ii)
Products of plasma extravasation (clotting factors, cellular products such as platelets and erythrocytes which release products including amines (5HT), peptides (bradykinin), and various lipidic acids (prostaglandins)).
(iii)
Innate immune cascade wherein given the chemoattractants present in the injury site, there will be a migration of inflammatory cells including neutrophils and macrophages. These contribute products such as myeloperoxidases, cytokines (TNF/IL1β), nerve growth factors (NGF), and serine proteases (trypsin).
(iv)
Terminal of primary afferent C fibers activated by the local milieu will lead to a local release of sP and CGRP which respectively cause vasodilation (erythema) and capillary leakiness (e.g., tissue swelling).
Importantly, these products have several effects on terminal function that are dependent upon the presence of the eponymous receptors on those terminals (e.g., trypsin activates proteinase-activated receptors: PARs; TNF) and the concentrations of the ligand (Fig. 1.6) [17].
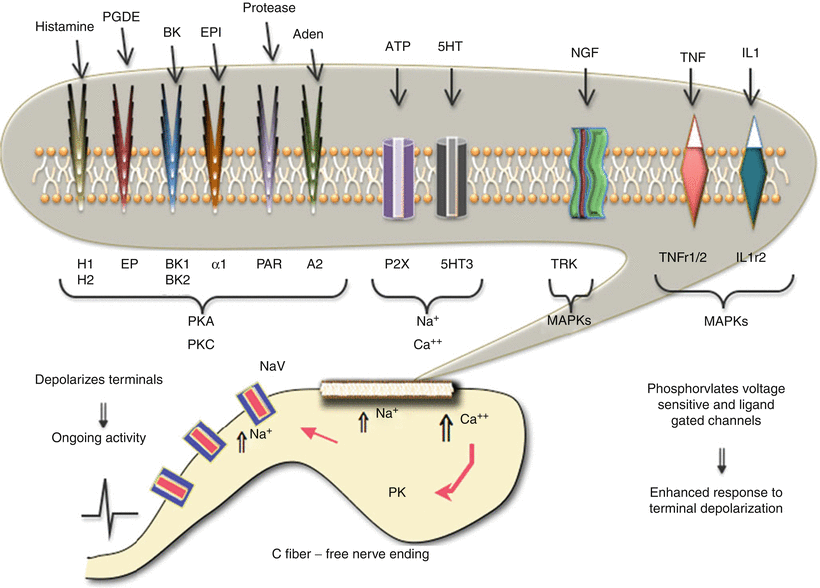

Fig. 1.6
This schematic provides an overview of the organization of events that initiate pain state after an injury to soft tissue. In the face of tissue injury, a variety of active products are released from local tissues, inflammatory cells, and the blood. These products exert a direct effect upon the small afferent terminal, free nerve endings, through specific receptors on the terminal. These receptors are coupled through a variety of second messengers which can lead to a local depolarization because of increased sodium and calcium influx. This leads to the activation of voltage-sensitive sodium channels (NaV) that initiate the regenerative action potential. In addition, the kinases and the increased intracellular calcium can initiate phosphorylation (PK) of channels and receptors, leading to an enhanced responsiveness of these channels and receptors. The net effect is to initiate an ongoing activity after the injuring stimulus has been removed and an increase in the discharge arising from any given stimulus
(i)
Activate the sensory terminal, increase intracellular calcium, and initiate a conducted action potential.
(ii)
Activate terminal kinases which serve to phosphorylate many membrane channels (e.g., sodium channels) and receptors (TRPV receptors) to increase their excitability. These actions are generally considered to result in spontaneous “afferent discharges” and to an enhanced responsiveness of the terminal to subsequent stimuli manifested by a left shift in the stimulus response curve for the sensory afferent. Overall, these properties are consistent with an ongoing pain stimulus and the ability of a given stimulus applied to that afferent in innervating the injured tissue to show a greater response (Fig. 1.2a).
It should be noted that these events are ubiquitous. This scenario has been demonstrated in numerous body systems, for example, cornea of the eye (sensitivity to light touch after abrasion), joint (pain of modest movement after inflammation of the knee), tooth pulp (sensory experience of cardiac-induced pressure changes in the tooth after inflammation of the pulp), and migraine (activation of the meningeal afferents which, like those in the tooth pulp, are not activated by normal mechanical movement or vascular pulsation). Indeed, think of any disease pathology described by the suffix “-itis” (Fig. 1.6).
Spinal Changes in Afferent Transmission Resulting from Tissue Injury
As reviewed above, acute activation of small afferents by extreme stimuli results in a spinal activation of dorsal horn neurons, the magnitude of which is proportional to the frequency (and identity) of the afferent input (Fig. 1.2e). Factors increasing that input-output relationship will cause a given stimulus to appear more intense (e.g., hyperalgesia). Conversely, factors reducing that function will cause a more intense stimulus to be encoded as less intense (e.g., analgesia). In the preceding section, it was appreciated that inflammation causes an enhanced response at the peripheral level. It is appreciated that there is also an enhanced response mediated in the spinal dorsal horn.
Central (Spinal) Facilitation
Animal research has demonstrated that repetitive afferent activation causes dorsal horn wide-dynamic-range (WDR) neurons to show evident signs of facilitation, labeled “windup” by Lorne Mendell and Patrick Wall (Fig. 1.4). This facilitation is characterized by following properties [18]:
(i)
High-frequency repetitive stimulation of C (but not A) fibers results in a progressively facilitated discharge of the WDR neurons.
(ii)
The receptive field of the WDR neuron showing windup was significantly expanded acutely following the conditioning afferent stimulation, for example, stimulation of an adjacent dermatome which hitherto did not activate that cell, would now lead to activity in that neuron (Fig. 1.7).
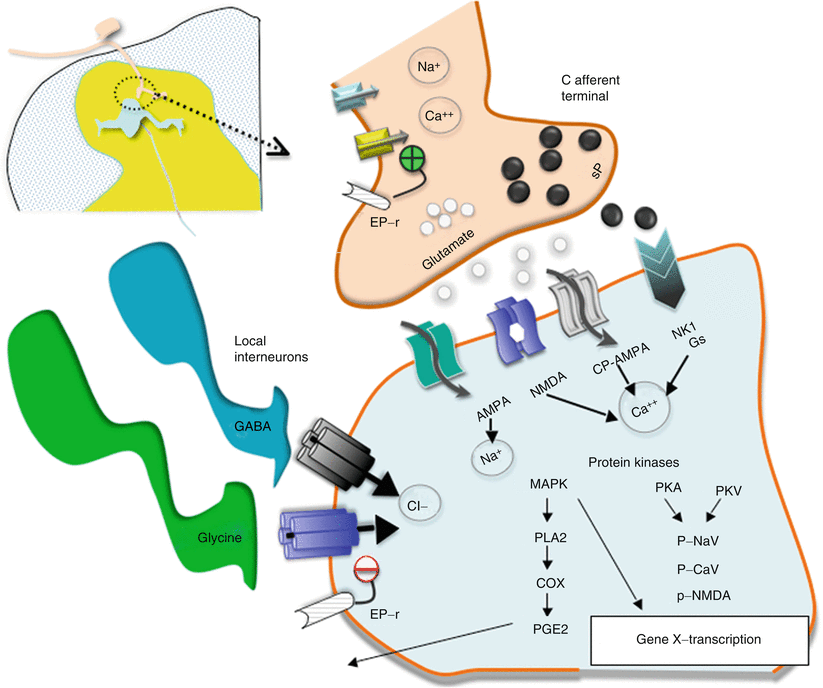

Fig. 1.7
This schematic provides an overview of the organization of the events transpiring at the level of the first-order synapse. (i) As indicated, the presynaptic effects of depolarization lead to opening of voltage-sensitive calcium and sodium channels with increases in intracellular sodium and calcium and mobilization and release of transmitters (sP and glutamate). (ii) These act upon eponymous receptors (see text), leading to depolarization and increase in intracellular calcium. (iii) Activation of kinases which phosphorylate a variety of channels and receptors activates intracellular enzyme cascades such as for PLA2 and increasing gene transcription. (iv) Release of products such as prostanoids (PGE2) which can act upon the local membrane through their eponymous receptors (EP-r) where presynaptically they enhance the opening of voltage-sensitive calcium channels and postsynaptically reduce the activity of glycine receptors. (v). As indicated in addition, the first-order synapse is regulated by inhibitor interneurons such as those release GABA and glycine. These interneurons can be activated by afferent collaterals and by descending pathways to downregulate the excitability of this synapse
Enhanced Response of WDR Neuron
The enhanced responsiveness of the cell was shown by intracellular recording to reflect a progressive and sustained (after termination of the stimulation) excitability of the neuron of the cell, rendering the membrane increasingly susceptible to even weak afferent inputs.
The enlarged receptive field can be explained by the ability of subliminal input coming from afferent input arising from an adjacent non-injured receptive field which was otherwise insufficient to activate a normally excitable cell.
Pharmacology of Central Facilitation
The enhanced excitability of dorsal horn neurons secondary to repetitive small afferent input reflects a series of complex mechanistic motifs that have a diverse pharmacology which will be briefly reviewed below. These can be broadly considered in terms of those systems which are (i) postsynaptic to the primary afferent and (ii) mediated by local neuronal networks, extraspinal networks, and nonneuronal networks. Examples will be reviewed below (Fig. 1.8).
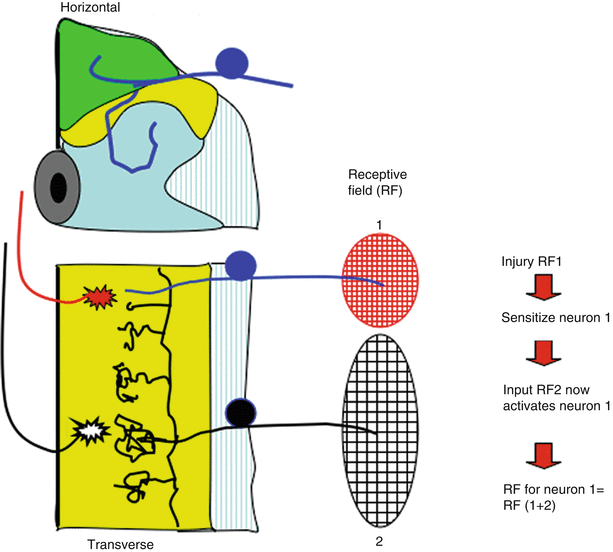

Fig. 1.8




Schematic presents the spinal cord in horizontal section (see Fig. 1.3). Receptive field of dorsal horn neuron depends upon the origin of its segmental input and the input from other segments, which can activate it. Thus, neuron 1 receives strong input from RF1 and very weak (ineffective) input from RF2. After injury in receptive field (RF) 1, neuron 1 becomes “sensitized.” Collateral input from RF2 normally is unable to initiate sufficient excitatory activity to activate neuron 1, but after sensitization, RF2 input is sufficient. Now, the RF of neuron 1 is effectively RF1 + RF2. Thus, local injury can by a spinal mechanism leads acutely to increased receptive fields such that stimuli applied to a non-injured RF can contribute to the post-tissue injury sensation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





